Pharmacovigilance in practice: erythropoiesis-stimulating agents
- PMID: 24890561
- PMCID: PMC4302692
- DOI: 10.1002/cam4.275
Pharmacovigilance in practice: erythropoiesis-stimulating agents
Abstract
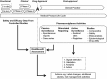
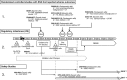
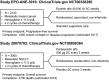
Pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or other problems related to medical products after they have been licensed for marketing. The purpose of PV is to advance the safe use of marketed medical products. Regulatory agencies and license holders collaborate to collect data reported by health care providers, patients, and the public as well as data from systematic reviews, meta-analyses, and individual clinical and nonclinical studies. They validate and analyze the data to determine whether safety signals exist, and if warranted, develop an action plan to mitigate the identified risk. Erythropoiesis-stimulating agents (ESAs) provide an example of how PV is applied in reality. Among other approved indications, ESAs may be used to treat anemia in patients with chemotherapy-induced anemia. ESAs increase hemoglobin levels and reduce the need for transfusions; they are also associated with a known increased risk of thromboembolic events. Starting in 2003, emerging data suggested that ESAs might reduce survival. As a result of PV activities by regulatory agencies and license holders, labeling for ESAs addresses these risks. Meta-analyses and individual clinical studies have confirmed that ESAs increase the risk of thromboembolic events, but when used as indicated, ESAs have not been shown to have a significant effect on survival or disease progression. Ongoing safety studies will provide additional data in the coming years to further clarify the risks and benefits of ESAs.
Keywords: Adverse event; chemotherapy induced anemia; erythropoiesis-stimulating agent; pharmacovigilance; safety signal.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Erythropoiesis-stimulating agents (ESAs): do they still have a role in chemotherapy-induced anemia (CIA)?Crit Rev Oncol Hematol. 2013 Aug;87(2):132-9. doi: 10.1016/j.critrevonc.2012.12.010. Epub 2013 Jan 26. Crit Rev Oncol Hematol. 2013. PMID: 23357249 Review.
-
Continued challenges with the use of erythropoiesis-stimulating agents in patients with cancer: perspectives and issues on policy-guided health care.Pharmacotherapy. 2008 May;28(5 Pt 2):1S-15S. doi: 10.1592/phco.28.5supp.1S. Pharmacotherapy. 2008. PMID: 18447704
-
Update on safety of ESAs in cancer-induced anemia.J Natl Compr Canc Netw. 2012 May;10(5):659-66. doi: 10.6004/jnccn.2012.0065. J Natl Compr Canc Netw. 2012. PMID: 22570294 Review.
-
Management of anemia in cancer patients.Future Oncol. 2011 Apr;7(4):507-17. doi: 10.2217/fon.11.24. Future Oncol. 2011. PMID: 21463140 Review.
-
Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update.Blood Adv. 2019 Apr 23;3(8):1197-1210. doi: 10.1182/bloodadvances.2018030387. Blood Adv. 2019. PMID: 30971397 Free PMC article. Review.
Cited by
-
The use of biosimilar medicines in oncology - position statement of the Brazilian Society of Clinical Oncology (SBOC).Braz J Med Biol Res. 2018 Jan 11;51(3):e7214. doi: 10.1590/1414-431X20177214. Braz J Med Biol Res. 2018. PMID: 29340530 Free PMC article.
-
A retrospective open-label uncontrolled study of Epoetin zeta on the treatment of chemotherapy-induced anemia in solid tumors.J Cancer Res Clin Oncol. 2017 Apr;143(4):717-725. doi: 10.1007/s00432-016-2339-5. Epub 2017 Jan 11. J Cancer Res Clin Oncol. 2017. PMID: 28078434 Free PMC article.
References
-
- European Medicines Agency (EMA) Guideline on good pharmacovigilance practices (GVP). Module V: risk management systems. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin... (accessed September 28, 2013)
-
- European Medicines Agency (EMA) and Heads of Medicines Agencies (HMA) Guideline on good pharmacovigilance practices (GVP) 2012. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_li.... (accessed March 5, 2013)
-
- U.S. Department of Health and Human Services FDA. 2005. Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER), Available at http://www.fda.gov/cder/guidance/index.htm. (accessed March 1, 2013)
-
- World Health Organization (WHO) 2006. The Safety of Medicines in Publich Health Programmes: pharmacovigilance an essential tool. Monitoring WCCfID, Available at http://www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharma.... (accessed March 1, 2013)
-
- Ebbers HC, Mantel-Teeuwisse AK, Moors EH, Schellekens H, Leufkens HG. Today's challenges in pharmacovigilance: what can we learn from epoetins? Drug Saf. 2011;34:273–287. doi: 10.2165/11586350-000000000-00000. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

