Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease
- PMID: 24890720
- PMCID: PMC4065844
- DOI: 10.4049/jimmunol.1301753
Aspirin activation of eosinophils and mast cells: implications in the pathogenesis of aspirin-exacerbated respiratory disease
Abstract
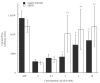
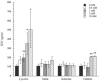
Reactions to aspirin and nonsteroidal anti-inflammatory drugs in patients with aspirin-exacerbated respiratory disease (AERD) are triggered when constraints upon activated eosinophils, normally supplied by PGE2, are removed secondary to cyclooxygenase-1 inhibition. However, the mechanism driving the concomitant cellular activation is unknown. We investigated the capacity of aspirin itself to provide this activation signal. Eosinophils were enriched from peripheral blood samples and activated with lysine ASA (LysASA). Parallel samples were stimulated with related nonsteroidal anti-inflammatory drugs. Activation was evaluated as Ca2+ flux, secretion of cysteinyl leukotrienes (CysLT), and eosinophil-derived neurotoxin (EDN) release. CD34+ progenitor-derived mast cells were also used to test the influence of aspirin on human mast cells with measurements of Ca2+ flux and PGD2 release. LysASA induced Ca2+ fluxes and EDN release, but not CysLT secretion from circulating eosinophils. There was no difference in the sensitivity or extent of activation between AERD and control subjects, and sodium salicylate was without effect. Like eosinophils, aspirin was able to activate human mast cells directly through Ca2+ flux and PGD2 release. AERD is associated with eosinophils maturing locally in a high IFN-γ milieu. As such, in additional studies, eosinophil progenitors were differentiated in the presence of IFN-γ prior to activation with aspirin. Eosinophils matured in the presence of IFN-γ displayed robust secretion of both EDN and CysLTs. These studies identify aspirin as the trigger of eosinophil and mast cell activation in AERD, acting in synergy with its ability to release cells from the anti-inflammatory constraints of PGE2.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures
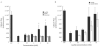
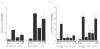
References
-
- Mascia K, Borish L, Patrie J, Hunt J, Phillips CD, Steinke JW. Chronic hyperplastic eosiniphilic sinusistis as a predictor of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2005;94:652–657. - PubMed
-
- Szczeklik A, Stevenson DD. Aspirin-induced asthma: Advances in pathogenesis and management. J Allergy Clin Immunol. 1999;104:5–13. - PubMed
-
- Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89:474–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

