Bovine γδ T cells are a major regulatory T cell subset
- PMID: 24890724
- PMCID: PMC4065783
- DOI: 10.4049/jimmunol.1303398
Bovine γδ T cells are a major regulatory T cell subset
Abstract
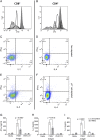
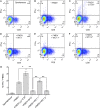
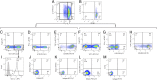
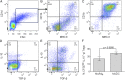
In humans and mice, γδ T cells represent <5% of the total circulating lymphocytes. In contrast, the γδ T cell compartment in ruminants accounts for 15-60% of the total circulating mononuclear lymphocytes. Despite the existence of CD4(+)CD25(high) Foxp3(+) T cells in the bovine system, these are neither anergic nor suppressive. We present evidence showing that bovine γδ T cells are the major regulatory T cell subset in peripheral blood. These γδ T cells spontaneously secrete IL-10 and proliferate in response to IL-10, TGF-β, and contact with APCs. IL-10-expressing γδ T cells inhibit Ag-specific and nonspecific proliferation of CD4(+) and CD8(+) T cells in vitro. APC subsets expressing IL-10 and TFG-β regulate proliferation of γδ T cells producing IL-10. We propose that γδ T cells are a major regulatory T cell population in the bovine system.
Copyright © 2014 The Authors.
Figures


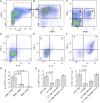

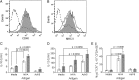
Similar articles
-
Identification and characterization of Foxp3(+) gammadelta T cells in mouse and human.Immunol Lett. 2009 Aug 15;125(2):105-13. doi: 10.1016/j.imlet.2009.06.005. Epub 2009 Jun 17. Immunol Lett. 2009. PMID: 19539651
-
Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus.J Immunol. 2011 Jun 15;186(12):6693-700. doi: 10.4049/jimmunol.1002776. Epub 2011 May 11. J Immunol. 2011. PMID: 21562160
-
Regulation of human CD4(+) alphabeta T-cell-receptor-positive (TCR(+)) and gammadelta TCR(+) T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor beta.Infect Immun. 1999 Dec;67(12):6461-72. doi: 10.1128/IAI.67.12.6461-6472.1999. Infect Immun. 1999. PMID: 10569764 Free PMC article.
-
γδ T-APCs: a novel tool for immunotherapy?Cell Mol Life Sci. 2011 Jul;68(14):2443-52. doi: 10.1007/s00018-011-0706-6. Epub 2011 May 15. Cell Mol Life Sci. 2011. PMID: 21573785 Free PMC article. Review.
-
Special features of γδ T cells in ruminants.Mol Immunol. 2021 Jun;134:161-169. doi: 10.1016/j.molimm.2021.02.028. Epub 2021 Mar 26. Mol Immunol. 2021. PMID: 33774521 Review.
Cited by
-
Temporal dynamics of intradermal cytokine response to tuberculin in Mycobacterium bovis BCG-vaccinated cattle using sampling microneedles.Sci Rep. 2021 Mar 29;11(1):7074. doi: 10.1038/s41598-021-86398-6. Sci Rep. 2021. PMID: 33782422 Free PMC article.
-
Advantages and Challenges of Differential Immune Cell Count Determination in Blood and Milk for Monitoring the Health and Well-Being of Dairy Cows.Vet Sci. 2022 May 27;9(6):255. doi: 10.3390/vetsci9060255. Vet Sci. 2022. PMID: 35737307 Free PMC article. Review.
-
Immunosuppression in Cows following Intramammary Infusion of Mycoplasma bovis.Infect Immun. 2020 Feb 20;88(3):e00521-19. doi: 10.1128/IAI.00521-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31843962 Free PMC article.
-
Recent Advances in Camel Immunology.Front Immunol. 2021 Jan 25;11:614150. doi: 10.3389/fimmu.2020.614150. eCollection 2020. Front Immunol. 2021. PMID: 33569060 Free PMC article.
-
To the Skin and Beyond: The Immune Response to African Trypanosomes as They Enter and Exit the Vertebrate Host.Front Immunol. 2020 Jun 12;11:1250. doi: 10.3389/fimmu.2020.01250. eCollection 2020. Front Immunol. 2020. PMID: 32595652 Free PMC article. Review.
References
-
- Komori H. K., Meehan T. F., Havran W. L. 2006. Epithelial and mucosal gamma delta T cells. Curr. Opin. Immunol. 18: 534–538. - PubMed
-
- Kabelitz D., Peters C., Wesch D., Oberg H. H. 2013. Regulatory functions of γδ T cells. Int. Immunopharmacol. 16: 382–387. - PubMed
-
- Davis W. C., Brown W. C., Hamilton M. J., Wyatt C. R., Orden J. A., Khalid A. M., Naessens J. 1996. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet. Immunol. Immunopathol. 52: 275–283. - PubMed
-
- Holm D., Fink D. R., Grønlund J., Hansen S., Holmskov U. 2009. Cloning and characterization of SCART1, a novel scavenger receptor cysteine-rich type I transmembrane molecule. Mol. Immunol. 46: 1663–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00001373/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001709/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F013590/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D001536/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001715/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

