Edwardsiella tarda-Induced cytotoxicity depends on its type III secretion system and flagellin
- PMID: 24891103
- PMCID: PMC4136207
- DOI: 10.1128/IAI.01065-13
Edwardsiella tarda-Induced cytotoxicity depends on its type III secretion system and flagellin
Abstract
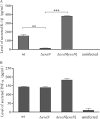
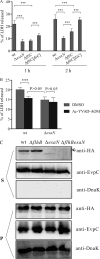
Many Gram-negative bacteria utilize a type III secretion system (T3SS) to translocate virulence proteins into host cells to cause diseases. In responding to infection, macrophages detect some of the translocated proteins to activate caspase-1-mediated cell death, called pyroptosis, and secretion of proinflammatory cytokines to control the infection. Edwardsiella tarda is a Gram-negative enteric pathogen that causes hemorrhagic septicemia in fish and both gastrointestinal and extraintestinal infections in humans. In this study, we report that the T3SS of E. tarda facilitates its survival and replication in murine bone marrow-derived macrophages, and E. tarda infection triggers pyroptosis of infected macrophages from mice and fish and increased secretion of the cytokine interleukin 1β in a T3SS-dependent manner. Deletion of the flagellin gene fliC of E. tarda results in decreased cytotoxicity for infected macrophages and does not attenuate its virulence in a fish model of infection, whereas upregulated expression of FliC in the fliC mutant strain reduces its virulence. We propose that the host controls E. tarda infection partially by detecting FliC translocated by the T3SS, whereas the bacteria downregulate the expression of FliC to evade innate immunity.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Identification and functional characterization of the novel Edwardsiella tarda effector EseJ.Infect Immun. 2015 Apr;83(4):1650-60. doi: 10.1128/IAI.02566-14. Epub 2015 Feb 9. Infect Immun. 2015. PMID: 25667268 Free PMC article.
-
Intramacrophage Infection Reinforces the Virulence of Edwardsiella tarda.J Bacteriol. 2016 Apr 28;198(10):1534-42. doi: 10.1128/JB.00978-15. Print 2016 May 15. J Bacteriol. 2016. PMID: 26953340 Free PMC article.
-
Edwardsiella tarda EscE (Orf13 Protein) Is a Type III Secretion System-Secreted Protein That Is Required for the Injection of Effectors, Secretion of Translocators, and Pathogenesis in Fish.Infect Immun. 2015 Oct 12;84(1):2-10. doi: 10.1128/IAI.00986-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26459509 Free PMC article.
-
Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish.Vet Res. 2012 Oct 4;43(1):67. doi: 10.1186/1297-9716-43-67. Vet Res. 2012. PMID: 23035843 Free PMC article. Review.
-
Edwardsiella tarda - virulence mechanisms of an emerging gastroenteritis pathogen.Microbes Infect. 2012 Jan;14(1):26-34. doi: 10.1016/j.micinf.2011.08.005. Epub 2011 Sep 1. Microbes Infect. 2012. PMID: 21924375 Review.
Cited by
-
Edwardsiella piscicida Enters Nonphagocytic Cells via a Macropinocytosis-Involved Hybrid Mechanism.J Bacteriol. 2019 Feb 11;201(5):e00548-18. doi: 10.1128/JB.00548-18. Print 2019 Mar 1. J Bacteriol. 2019. PMID: 30530518 Free PMC article.
-
Unraveling and characterization of novel T3SS effectors in Edwardsiella piscicida.mSphere. 2023 Oct 24;8(5):e0034623. doi: 10.1128/msphere.00346-23. Epub 2023 Aug 29. mSphere. 2023. PMID: 37642418 Free PMC article.
-
Identification and validation of sRNAs in Edwardsiella tarda S08.PLoS One. 2017 Mar 7;12(3):e0172783. doi: 10.1371/journal.pone.0172783. eCollection 2017. PLoS One. 2017. PMID: 28267754 Free PMC article.
-
Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling.Nature. 2015 Oct 29;526(7575):666-71. doi: 10.1038/nature15541. Epub 2015 Sep 16. Nature. 2015. PMID: 26375259
-
Proteus faecis: a potentially pathogenic bacterium isolated from the freshwater Yangtze finless porpoise.Antonie Van Leeuwenhoek. 2024 Sep 21;118(1):7. doi: 10.1007/s10482-024-02023-2. Antonie Van Leeuwenhoek. 2024. PMID: 39305395
References
-
- Yousuf RM, How SH, Amran M, Hla KT, Shah A, Francis A. 2006. Edwardsiella tarda septicemia with underlying multiple liver abscesses. Malays. J. Pathol. 28:49–53. - PubMed
-
- Okuda J, Arikawa Y, Takeuchi Y, Mahmoud MM, Suzaki E, Kataoka K, Suzuki T, Okinaka Y, Nakai T. 2006. Intracellular replication of Edwardsiella tarda in murine macrophage is dependent on the type III secretion system and induces an up-regulation of anti-apoptotic NF-kappaB target genes protecting the macrophage from staurosporine-induced apoptosis. Microb. Pathog. 41:226–240. 10.1016/j.micpath.2006.08.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

