Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis
- PMID: 24891104
- PMCID: PMC4136217
- DOI: 10.1128/IAI.01746-14
Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis
Abstract
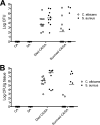
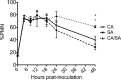
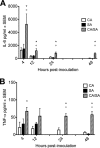
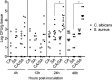
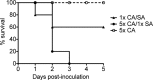
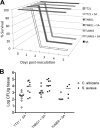
Intra-abdominal polymicrobial infections cause significant morbidity and mortality. An established experimental mouse model of Staphylococcus aureus-Candida albicans intra-abdominal infection results in ∼60% mortality within 48 h postinoculation, concomitant with amplified local inflammatory responses, while monomicrobial infections are avirulent. The purpose of this study was to characterize early local and systemic innate responses during coinfection and determine the role of C. albicans morphogenesis in lethality, a trait involved in virulence and physical interaction with S. aureus. Local and systemic proinflammatory cytokines were significantly elevated during coinfection at early time points (4 to 12 h) compared to those in monoinfection. In contrast, microbial burdens in the organs and peritoneal lavage fluid were similar between mono- and coinfected animals through 24 h, as was peritoneal neutrophil infiltration. After optimizing the model for 100% mortality within 48 h, using 3.5 × 10(7) C. albicans (5× increase), coinfection with C. albicans yeast-locked or hypha-locked mutants showed similar mortality, dissemination, and local and systemic inflammation to the isogenic control. However, coinfection with the yeast-locked C. albicans mutant given intravenously (i.v.) and S. aureus given intraperitoneally (i.p.) failed to induce mortality. These results suggest a unique intra-abdominal interaction between the host and C. albicans-S. aureus that results in strong inflammatory responses, dissemination, and lethal sepsis, independent of C. albicans morphogenesis.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Heemken R, Gandawidjaja L, Hau T. 1997. Peritonitis: pathophysiology and local defense mechanisms. Hepatogastroenterology 44:927–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

