Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia
- PMID: 24891325
- PMCID: PMC4284068
- DOI: 10.1136/thoraxjnl-2013-204980
Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia
Abstract
Background: Human bone marrow-derived mesenchymal stem (stromal) cells (hMSCs) improve survival in mouse models of acute respiratory distress syndrome (ARDS) and reduce pulmonary oedema in a perfused human lung preparation injured with Escherichia coli bacteria. We hypothesised that clinical grade hMSCs would reduce the severity of acute lung injury (ALI) and would be safe in a sheep model of ARDS.
Methods: Adult sheep (30-40 kg) were surgically prepared. After 5 days of recovery, ALI was induced with cotton smoke insufflation, followed by instillation of live Pseudomonas aeruginosa (2.5×10(11) CFU) into both lungs under isoflurane anaesthesia. Following the injury, sheep were ventilated, resuscitated with lactated Ringer's solution and studied for 24 h. The sheep were randomly allocated to receive one of the following treatments intravenously over 1 h in one of the following groups: (1) control, PlasmaLyte A, n=8; (2) lower dose hMSCs, 5×10(6) hMSCs/kg, n=7; and (3) higher-dose hMSCs, 10×10(6) hMSCs/kg, n=4.
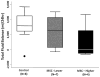
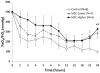
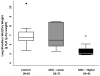
Results: By 24 h, the PaO2/FiO2 ratio was significantly improved in both hMSC treatment groups compared with the control group (control group: PaO2/FiO2 of 97±15 mm Hg; lower dose: 288±55 mm Hg (p=0.003); higher dose: 327±2 mm Hg (p=0.003)). The median lung water content was lower in the higher-dose hMSC-treated group compared with the control group (higher dose: 5.0 g wet/g dry [IQR 4.9-5.8] vs control: 6.7 g wet/g dry [IQR 6.4-7.5] (p=0.01)). The hMSCs had no adverse effects.
Conclusions: Human MSCs were well tolerated and improved oxygenation and decreased pulmonary oedema in a sheep model of severe ARDS.
Trail registration number: NCT01775774 for Phase 1. NCT02097641 for Phase 2.
Keywords: ARDS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Figures

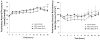
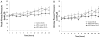
References
-
- Ware LB, Matthay MA. Acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. - PubMed
-
- Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–6. - PubMed
-
- ARDS definition task force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. - PubMed
-
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
