FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia
- PMID: 24891437
- PMCID: PMC4209879
- DOI: 10.1093/ndt/gfu201
FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia
Abstract
Background: The optimal iron therapy regimen in patients with non-dialysis-dependent chronic kidney disease (CKD) is unknown.
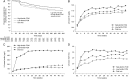
Methods: Ferinject® assessment in patients with Iron deficiency anaemia and Non-Dialysis-dependent Chronic Kidney Disease (FIND-CKD) was a 56-week, open-label, multicentre, prospective and randomized study of 626 patients with non-dialysis-dependent CKD, anaemia and iron deficiency not receiving erythropoiesis-stimulating agents (ESAs). Patients were randomized (1:1:2) to intravenous (IV) ferric carboxymaltose (FCM), targeting a higher (400-600 µg/L) or lower (100-200 µg/L) ferritin or oral iron therapy. The primary end point was time to initiation of other anaemia management (ESA, other iron therapy or blood transfusion) or haemoglobin (Hb) trigger of two consecutive values <10 g/dL during Weeks 8-52.
Results: The primary end point occurred in 36 patients (23.5%), 49 patients (32.2%) and 98 patients (31.8%) in the high-ferritin FCM, low-ferritin FCM and oral iron groups, respectively [hazard ratio (HR): 0.65; 95% confidence interval (CI): 0.44-0.95; P = 0.026 for high-ferritin FCM versus oral iron]. The increase in Hb was greater with high-ferritin FCM versus oral iron (P = 0.014) and a greater proportion of patients achieved an Hb increase ≥1 g/dL with high-ferritin FCM versus oral iron (HR: 2.04; 95% CI: 1.52-2.72; P < 0.001). Rates of adverse events and serious adverse events were similar in all groups.
Conclusions: Compared with oral iron, IV FCM targeting a ferritin of 400-600 µg/L quickly reached and maintained Hb level, and delayed and/or reduced the need for other anaemia management including ESAs. Within the limitations of this trial, no renal toxicity was observed, with no difference in cardiovascular or infectious events.
Clinicaltrialsgov number: NCT00994318.
Keywords: anaemia; chronic kidney disease.
© The Author 2014. Published by Oxford University Press on behalf of ERA-EDTA.
Figures

Comment in
-
Anaemia: FIND-CKD: intravenous iron in predialysis CKD.Nat Rev Nephrol. 2014 Sep;10(9):488-9. doi: 10.1038/nrneph.2014.139. Epub 2014 Aug 5. Nat Rev Nephrol. 2014. PMID: 25092153 No abstract available.
References
-
- K idney Disease Improving Global Outcomes (KDIGO). Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:292–298.
-
- Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. - PubMed
-
- Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. - PubMed
-
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. - PubMed
-
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

