Genomic determinants of gene regulation by 1,25-dihydroxyvitamin D3 during osteoblast-lineage cell differentiation
- PMID: 24891508
- PMCID: PMC4094065
- DOI: 10.1074/jbc.M114.578104
Genomic determinants of gene regulation by 1,25-dihydroxyvitamin D3 during osteoblast-lineage cell differentiation
Abstract
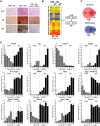
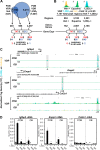
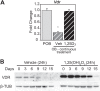
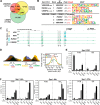
The biological effects of 1α,25-dihydroxyvitamin D3 (1,25 (OH)2D3) on osteoblast differentiation and function differ significantly depending upon the cellular state of maturation. To explore this phenomenon mechanistically, we examined the impact of 1,25(OH)2D3 on the transcriptomes of both pre-osteoblastic (POBs) and differentiated osteoblastic (OBs) MC3T3-E1 cells, and assessed localization of the vitamin D receptor (VDR) at sites of action on a genome-scale using ChIP sequence analysis. We observed that the 1,25(OH)2D3-induced transcriptomes of POBs and OBs were quantitatively and qualitatively different, supporting not only the altered biology observed but the potential for a change in VDR interaction at the genome as well. This idea was confirmed through discovery that VDR cistromes in POBs and OBs were also strikingly different. Depletion of VDR-binding sites in OBs, due in part to reduced VDR expression, was the likely cause of the loss of VDR-target gene interaction. Continued novel regulation by 1,25(OH)2D3, however, suggested that factors in addition to the VDR might also be involved. Accordingly, we show that transcriptomic modifications are also accompanied by changes in genome binding of the master osteoblast regulator RUNX2 and the chromatin remodeler CCAAT/enhancer-binding protein β. Importantly, genome occupancy was also highlighted by the presence of epigenetic enhancer signatures that were selectively changed in response to both differentiation and 1,25(OH)2D3. The impact of VDR, RUNX2, and C/EBPβ on osteoblast differentiation is exemplified by their actions at the Runx2 and Sp7 gene loci. We conclude that each of these mechanisms may contribute to the diverse actions of 1,25(OH)2D3 on differentiating osteoblasts.
Keywords: CCAAT/Enhancer-binding Protein (C/EBP); ChIP-sequencing (ChIP-seq); Chromatin Modification; Osteoblast; Transcription Enhancer; Vitamin D.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Lieben L., Carmeliet G., Masuyama R. (2011) Calcemic actions of vitamin D: effects on the intestine, kidney and bone. Best Pract. Res. Clin. Endocrinol. Metab. 25, 561–572 - PubMed
-
- Plum L. A., DeLuca H. F. (2010) Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 9, 941–955 - PubMed
-
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

