Cotyledon-Generated Auxin Is Required for Shade-Induced Hypocotyl Growth in Brassica rapa
- PMID: 24891610
- PMCID: PMC4081337
- DOI: 10.1104/pp.114.241844
Cotyledon-Generated Auxin Is Required for Shade-Induced Hypocotyl Growth in Brassica rapa
Abstract
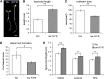
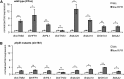
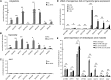
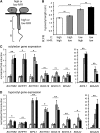
Plant architecture is optimized for the local light environment. In response to foliar shade or neighbor proximity (low red to far-red light), some plant species exhibit shade-avoiding phenotypes, including increased stem and hypocotyl growth, which increases the likelihood of outgrowing competitor plants. If shade persists, early flowering and the reallocation of growth resources to stem elongation ultimately affect the yield of harvestable tissues in crop species. Previous studies have shown that hypocotyl growth in low red to far-red shade is largely dependent on the photoreceptor phytochrome B and the phytohormone auxin. However, where shade is perceived in the plant and how auxin regulates growth spatially are less well understood. Using the oilseed and vegetable crop species Brassica rapa, we show that the perception of low red to far-red shade by the cotyledons triggers hypocotyl cell elongation and auxin target gene expression. Furthermore, we find that following shade perception, elevated auxin levels occur in a basipetal gradient away from the cotyledons and that this is coincident with a gradient of auxin target gene induction. These results show that cotyledon-generated auxin regulates hypocotyl elongation. In addition, we find in mature B. rapa plants that simulated shade does not affect seed oil composition but may affect seed yield. This suggests that in field settings where mutual shading between plants may occur, a balance between plant density and seed yield per plant needs to be achieved for maximum oil yield, while oil composition might remain constant.
© 2014 American Society of Plant Biologists. All Rights Reserved.
Figures




Similar articles
-
Hormonal Regulation in Shade Avoidance.Front Plant Sci. 2017 Sep 4;8:1527. doi: 10.3389/fpls.2017.01527. eCollection 2017. Front Plant Sci. 2017. PMID: 28928761 Free PMC article. Review.
-
Comparative phenotypic and transcriptomic analyses unravel conserved and distinct mechanisms underlying shade avoidance syndrome in Brassicaceae vegetables.BMC Genomics. 2021 Oct 25;22(1):760. doi: 10.1186/s12864-021-08076-1. BMC Genomics. 2021. PMID: 34696740 Free PMC article.
-
Arabidopsis COP1 and SPA genes are essential for plant elongation but not for acceleration of flowering time in response to a low red light to far-red light ratio.Plant Physiol. 2012 Dec;160(4):2015-27. doi: 10.1104/pp.112.207233. Epub 2012 Oct 23. Plant Physiol. 2012. PMID: 23093358 Free PMC article.
-
Unraveling the Dynamic Integration of Auxin, Brassinosteroid and Gibberellin in Early Shade-Induced Hypocotyl Elongation.Phenomics. 2022 Feb 13;2(2):119-129. doi: 10.1007/s43657-022-00044-3. eCollection 2022 Apr. Phenomics. 2022. PMID: 36939748 Free PMC article.
-
Photoreceptors Regulate Plant Developmental Plasticity through Auxin.Plants (Basel). 2020 Jul 24;9(8):940. doi: 10.3390/plants9080940. Plants (Basel). 2020. PMID: 32722230 Free PMC article. Review.
Cited by
-
Organ-specific transcriptional regulation by HFR1 and HY5 in response to shade in Arabidopsis.Front Plant Sci. 2024 Jul 31;15:1430639. doi: 10.3389/fpls.2024.1430639. eCollection 2024. Front Plant Sci. 2024. PMID: 39145190 Free PMC article.
-
Progress of Research on the Regulatory Pathway of the Plant Shade-Avoidance Syndrome.Front Plant Sci. 2020 Apr 15;11:439. doi: 10.3389/fpls.2020.00439. eCollection 2020. Front Plant Sci. 2020. PMID: 32351535 Free PMC article. Review.
-
Spatial regulation of plant hormone action.J Exp Bot. 2023 Oct 13;74(19):6089-6103. doi: 10.1093/jxb/erad244. J Exp Bot. 2023. PMID: 37401809 Free PMC article. Review.
-
Multiomics analysis of gibberellin involved in far-red light-regulated internode elongation in cucumber seedlings.Plant Cell Rep. 2025 Sep 6;44(10):208. doi: 10.1007/s00299-025-03598-4. Plant Cell Rep. 2025. PMID: 40913742
-
Species-specific PHYTOCHROME-INTERACTING FACTOR utilization in the plant morphogenetic response to environmental stimuli.Plant Cell. 2025 May 9;37(5):koaf048. doi: 10.1093/plcell/koaf048. Plant Cell. 2025. PMID: 40085779 Free PMC article.
References
-
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23: 439–446 - PubMed
-
- Ballaré CL. (2009) Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ 32: 713–725 - PubMed
-
- Ballare CL, Scopel AL, Sanchez RA. (1997) Foraging for light: photosensory ecology and agricultural implications. Plant Cell Environ 20: 820–825
-
- Black M, Shuttleworth JE. (1974) The role of the cotyledons in the photocontrol of hypocotyl extension in Cucumis sativus L. Planta 117: 57–66 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

