Lung epithelial stem cells and their niches: Fgf10 takes center stage
- PMID: 24891877
- PMCID: PMC4041638
- DOI: 10.1186/1755-1536-7-8
Lung epithelial stem cells and their niches: Fgf10 takes center stage
Abstract
Throughout life adult animals crucially depend on stem cell populations to maintain and repair their tissues to ensure life-long organ function. Stem cells are characterized by their capacity to extensively self-renew and give rise to one or more differentiated cell types. These powerful stem cell properties are key to meet the changing demand for tissue replacement during normal lung homeostasis and regeneration after lung injury. Great strides have been made over the last few years to identify and characterize lung epithelial stem cells as well as their lineage relationships. Unfortunately, knowledge on what regulates the behavior and fate specification of lung epithelial stem cells is still limited, but involves communication with their microenvironment or niche, a local tissue environment that hosts and influences the behaviors or characteristics of stem cells and that comprises other cell types and extracellular matrix. As such, an intimate and dynamic epithelial-mesenchymal cross-talk, which is also essential during lung development, is required for normal homeostasis and to mount an appropriate regenerative response after lung injury. Fibroblast growth factor 10 (Fgf10) signaling in particular seems to be a well-conserved signaling pathway governing epithelial-mesenchymal interactions during lung development as well as between different adult lung epithelial stem cells and their niches. On the other hand, disruption of these reciprocal interactions leads to a dysfunctional epithelial stem cell-niche unit, which may culminate in chronic lung diseases such as chronic obstructive pulmonary disease (COPD), chronic asthma and idiopathic pulmonary fibrosis (IPF).
Figures

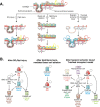
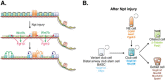

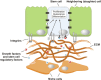
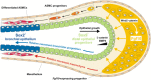
References
-
- Eisenhauer P, Earle B, Loi R, Sueblinvong V, Goodwin M, Allen GB, Lundblad L, Mazan MR, Hoffman AM, Weiss DJ. Endogenous distal airway progenitor cells, lung mechanics, and disproportionate lobar growth following long-term postpneumonectomy in mice. Stem Cells. 2013;31:1330–1339. doi: 10.1002/stem.1377. - DOI - PMC - PubMed
-
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

