The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases
- PMID: 24891994
- PMCID: PMC4030606
- DOI: 10.7453/gahmj.2014.017
The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases
Abstract
Tauroursodeoxycholic acid (TUDCA) is the taurine conjugate of ursodeoxycholic acid (UDCA), a US Food and Drug Administration-approved hydrophilic bile acid for the treatment of certain cholestatic liver diseases. There is a growing body of research on the mechanism(s) of TUDCA and its potential therapeutic effect on a wide variety of non-liver diseases. Both UDCA and TUDCA are potent inhibitors of apoptosis, in part by interfering with the upstream mitochondrial pathway of cell death, inhibiting oxygen-radical production, reducing endoplasmic reticulum (ER) stress, and stabilizing the unfolded protein response (UPR). Several studies have demonstrated that TUDCA serves as an anti-apoptotic agent for a number of neurodegenerative diseases, including amyotrophic lateral sclerosis, Alzheimer's disease, Parkinson's disease, and Huntington's disease. In addition, TUDCA plays an important role in protecting against cell death in certain retinal disorders, such as retinitis pigmentosa. It has been shown to reduce ER stress associated with elevated glucose levels in diabetes by inhibiting caspase activation, up-regulating the UPR, and inhibiting reactive oxygen species. Obesity, stroke, acute myocardial infarction, spinal cord injury, and a long list of acute and chronic non-liver diseases associated with apoptosis are all potential therapeutic targets for T/UDCA. A growing number of pre-clinical and clinical studies underscore the potential benefit of this simple, naturally occurring bile acid, which has been used in Chinese medicine for more than 3000 years.
Keywords: Apoptosis; bile acids; chaperone; cytoprotection; endoplasmic reticulum stress; misfolded proteins; neurodegenerative diseases; tauroursodeoxy-cholic acid (TUDCA); unfolded protein response; ursodeoxycholic acid (UDCA).
Figures
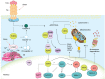

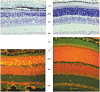
References
-
- Omura T, Asari M, Yamamoto J, et al.Sodium tauroursodeoxycholate prevents paraquat-induced cell death by suppressing endoplasmic reticulum stress responses in human lung epithelial A549 cells. Biochem Biophys Res Commun. 2013;432(4):689–94 - PubMed
-
- Beuers U. β1 integrin is a long-sought sensor for tauroursodeoxycholic acid. Hepatology. 2013;57(3):867–9 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

