Inhibition of neutral sphingomyelinases in skeletal muscle attenuates fatty-acid induced defects in metabolism and stress
- PMID: 24892004
- PMCID: PMC4039661
- DOI: 10.1186/2193-1801-3-255
Inhibition of neutral sphingomyelinases in skeletal muscle attenuates fatty-acid induced defects in metabolism and stress
Abstract
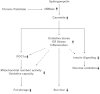
Background: Chronic metabolic overload leads to insulin resistance in a variety of tissues. It has been shown that exposure to saturated fatty acid palmitate can cause insulin resistance in skeletal muscle cells. Fatty acid induced synthesis of ceramide is considered to be one of the major causes for insulin resistance. Both de novo synthesis and sphingomyelin hydrolysis by sphingomyelinase are implicated for ceramide generation. Aim of this study was to evaluate the impact of neutral sphingomyelinase (nSMase) inhibition on saturated fatty acid induced lipotoxicity and insulin resistance in skeletal muscle myotubes.
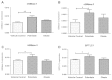
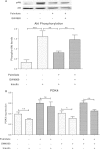
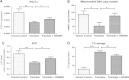
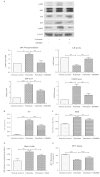
Results: Treatment of saturated fatty acid (palmitate) but not unsaturated fatty acid (oleate) caused an up-regulation in expression of various nSMase genes which are associated with ceramide synthesis through the salvage pathway. Inhibition of nSMase by a pharmacological inhibitor (GW4869) partially reverted the palmitate induced insulin resistance in C2C12 myotubes. Inhibition of nSMase improved metabolic functions of myotubes as measured by improved oxidative capacity in terms of increased mitochondrial number, PGC1α expression and ATP levels with concomitant decrease in intramyocellular triglyceride levels. Palmitate induced inflammatory response was also reduced by nSMase inhibitor. GW4869 treatment reduced palmitate induced oxidative and endoplasmic reticulum stress and improved cell survival.
Conclusion: In this study, we provide evidences that inhibition of nSMase can protect skeletal muscles from saturated fatty acid induced insulin resistance, metabolic dysfunction, cellular stress and inflammation.
Keywords: C2C12 myotubes; Cellular stress; Ceramide; Insulin resistance; Oxidative capacity; Sphingomyelinase; T2DM.
Figures
Similar articles
-
Palmitate and oleate exert differential effects on insulin signalling and glucose uptake in human skeletal muscle cells.Endocr Connect. 2017 Jul;6(5):331-339. doi: 10.1530/EC-17-0039. Epub 2017 Jun 5. Endocr Connect. 2017. PMID: 28584168 Free PMC article.
-
Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes.Arch Biochem Biophys. 2003 Nov 15;419(2):101-9. doi: 10.1016/j.abb.2003.08.020. Arch Biochem Biophys. 2003. PMID: 14592453
-
Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate.J Biol Chem. 1999 Aug 20;274(34):24202-10. doi: 10.1074/jbc.274.34.24202. J Biol Chem. 1999. PMID: 10446195
-
Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid.J Cell Physiol. 2007 Apr;211(1):244-52. doi: 10.1002/jcp.20922. J Cell Physiol. 2007. PMID: 17219404
-
Sphingomyelinases and Liver Diseases.Biomolecules. 2020 Oct 30;10(11):1497. doi: 10.3390/biom10111497. Biomolecules. 2020. PMID: 33143193 Free PMC article. Review.
Cited by
-
Neutral Sphingomyelinase-2 (NSM 2) Controls T Cell Metabolic Homeostasis and Reprogramming During Activation.Front Mol Biosci. 2020 Sep 4;7:217. doi: 10.3389/fmolb.2020.00217. eCollection 2020. Front Mol Biosci. 2020. PMID: 33088808 Free PMC article.
-
The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms.Inflamm Res. 2019 Nov;68(11):915-932. doi: 10.1007/s00011-019-01273-5. Epub 2019 Jul 30. Inflamm Res. 2019. PMID: 31363792 Free PMC article. Review.
-
β2-Adrenergic Regulation of the Neuromuscular Transmission and Its Lipid-Dependent Switch.Mol Neurobiol. 2024 Sep;61(9):6805-6821. doi: 10.1007/s12035-024-03991-2. Epub 2024 Feb 14. Mol Neurobiol. 2024. PMID: 38353924
-
Sphingolipid changes do not underlie fatty acid-evoked GLUT4 insulin resistance nor inflammation signals in muscle cells.J Lipid Res. 2018 Jul;59(7):1148-1163. doi: 10.1194/jlr.M080788. Epub 2018 May 23. J Lipid Res. 2018. PMID: 29794037 Free PMC article.
-
Neutral sphingomyelinase-2 and cardiometabolic diseases.Obes Rev. 2021 Aug;22(8):e13248. doi: 10.1111/obr.13248. Epub 2021 Mar 18. Obes Rev. 2021. PMID: 33738905 Free PMC article. Review.
References
-
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. - DOI - PubMed
-
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

