Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications
- PMID: 24892144
- PMCID: PMC4047642
- DOI: 10.1016/j.jaci.2014.02.006
Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications
Abstract
Background: Although recent studies have identified the presence of phenotypic clusters in asthmatic patients, the clinical significance and temporal stability of these clusters have not been explored.
Objective: Our aim was to examine the clinical relevance and temporal stability of phenotypic clusters in children with asthma.
Methods: We applied spectral clustering to clinical data from 1041 children with asthma participating in the Childhood Asthma Management Program. Posttreatment randomization follow-up data collected over 48 months were used to determine the effect of these clusters on pulmonary function and treatment response to inhaled anti-inflammatory medication.
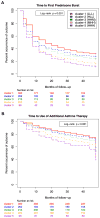
Results: We found 5 reproducible patient clusters that could be differentiated on the basis of 3 groups of features: atopic burden, degree of airway obstruction, and history of exacerbation. Cluster grouping predicted long-term asthma control, as measured by the need for oral prednisone (P < .0001) or additional controller medications (P = .001), as well as longitudinal differences in pulmonary function (P < .0001). We also found that the 2 clusters with the highest rates of exacerbation had different responses to inhaled corticosteroids when compared with the other clusters. One cluster demonstrated a positive response to both budesonide (P = .02) and nedocromil (P = .01) compared with placebo, whereas the other cluster demonstrated minimal responses to both budesonide (P = .12) and nedocromil (P = .56) compared with placebo.
Conclusion: Phenotypic clustering can be used to identify longitudinally consistent and clinically relevant patient subgroups, with implications for targeted therapeutic strategies and clinical trials design.
Conflict of interest statement
Disclosure of potential conflict of interest: A. L. Fuhlbrigge has received research support from the National Heart, Lung, and Blood Institute (NHLBI) and the Agency for Healthcare and Research Quality and has received personal fees from GlaxoSmithKline, Merck, Icon Medical Imaging, Lovelace Respiratory Research Institute, Scientific Therapeutics, and the American Academy of Allergy, Asthma & Immunology. R. S. Zeiger has received research support from the NHLBI, Genentech, GlaxoSmithKline, Aerocrine, Merck, MedImmune, and Thermo Fisher; is on the Research Advisory Board for DBV Technologies; has consultant arrangements with GlaxoSmithKline, Genentech, Novartis, the NHLBI/Penn State, Aerocrine, and AstraZeneca; and has stock/stock options in DBV Technologies. S. T. Weiss has consultant arrangements with Novartis. The rest of the authors declare that they have no relevant conflicts of interest.
Figures


References
-
- National Asthma Education and Prevention Program: expert panel report III: guidelines for the diagnosis and management of asthma. Bethesda: National Heart, Lung, and Blood Institute; 2007.
-
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. - PubMed
-
- Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–63. - PubMed
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL093076/HL/NHLBI NIH HHS/United States
- M01 RR002719/RR/NCRR NIH HHS/United States
- N01-HR-16044/HR/NHLBI NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- N01 HR016046/HL/NHLBI NIH HHS/United States
- N01-HR-16045/HR/NHLBI NIH HHS/United States
- M01RR02719-14/RR/NCRR NIH HHS/United States
- R01-086601/PHS HHS/United States
- N01 HR016045/HL/NHLBI NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- R01 HL086601/HL/NHLBI NIH HHS/United States
- N01-HR-16047/HR/NHLBI NIH HHS/United States
- N01-HR-16046/HR/NHLBI NIH HHS/United States
- N01 HR016047/HL/NHLBI NIH HHS/United States
- N01-HR-16049/HR/NHLBI NIH HHS/United States
- RR00036/RR/NCRR NIH HHS/United States
- R01-093076/PHS HHS/United States
- N01-HR-16051/HR/NHLBI NIH HHS/United States
- RC2 HL101543/HL/NHLBI NIH HHS/United States
- N01-HR-16048/HR/NHLBI NIH HHS/United States
- U01 HL065899/HL/NHLBI NIH HHS/United States
- N01 HR016044/HL/NHLBI NIH HHS/United States
- N01-HR-16052/HR/NHLBI NIH HHS/United States
- N01 HR016050/HL/NHLBI NIH HHS/United States
- R37 HL066289/HL/NHLBI NIH HHS/United States
- N01 HR016051/HL/NHLBI NIH HHS/United States
- R01 HL118455/HL/NHLBI NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- N01 HR016049/HL/NHLBI NIH HHS/United States
- N01-HR-16050/HR/NHLBI NIH HHS/United States
- N01 HR016052/HL/NHLBI NIH HHS/United States
- RC2-HL101543/HL/NHLBI NIH HHS/United States
- N01 HR016048/HL/NHLBI NIH HHS/United States
- M01RR00051/RR/NCRR NIH HHS/United States
- M01RR0099718-24/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

