Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer
- PMID: 24892425
- PMCID: PMC4043882
- DOI: 10.1371/journal.pone.0098623
Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer
Retraction in
-
Retraction: Macrophage Colony-Stimulating Factor Augments Tie2-Expressing Monocyte Differentiation, Angiogenic Function, and Recruitment in a Mouse Model of Breast Cancer.PLoS One. 2020 Jun 17;15(6):e0235096. doi: 10.1371/journal.pone.0235096. eCollection 2020. PLoS One. 2020. PMID: 32555748 Free PMC article. No abstract available.
Abstract
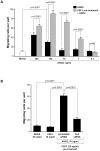
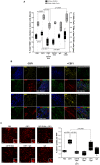
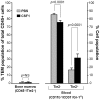
Reports demonstrate the role of M-CSF (CSF1) in tumor progression in mouse models as well as the prognostic value of macrophage numbers in breast cancer patients. Recently, a subset of CD14+ monocytes expressing the Tie2 receptor, once thought to be predominantly expressed on endothelial cells, has been characterized. We hypothesized that increased levels of CSF1 in breast tumors can regulate differentiation of Tie2- monocytes to a Tie2+ phenotype. We treated CD14+ human monocytes with CSF1 and found a significant increase in CD14+/Tie2+ positivity. To understand if CSF1-induced Tie2 expression on these cells improved their migratory ability, we pre-treated CD14+ monocytes with CSF1 and used Boyden chemotaxis chambers to observe enhanced response to angiopoietin-2 (ANG2), the chemotactic ligand for the Tie2 receptor. We found that CSF1 pre-treatment significantly augmented chemotaxis and that Tie2 receptor upregulation was responsible as siRNA targeting Tie2 receptor abrogated this effect. To understand any augmented angiogenic effect produced by treating these cells with CSF1, we cultured human umbilical vein endothelial cells (HUVECs) with conditioned supernatants from CSF1-pre-treated CD14+ monocytes for a tube formation assay. While supernatants from CSF1-pre-treated TEMs increased HUVEC branching, a neutralizing antibody against the CSF1R abrogated this activity, as did siRNA against the Tie2 receptor. To test our hypothesis in vivo, we treated PyMT tumor-bearing mice with CSF1 and observed an expansion in the TEM population relative to total F4/80+ cells, which resulted in increased angiogenesis. Investigation into the mechanism of Tie2 receptor upregulation on CD14+ monocytes by CSF1 revealed a synergistic contribution from the PI3 kinase and HIF pathways as the PI3 kinase inhibitor LY294002, as well as HIF-1α-deficient macrophages differentiated from the bone marrow of HIF-1αfl/fl/LysMcre mice, diminished CSF1-stimulated Tie2 receptor expression.
Conflict of interest statement
Figures



Similar articles
-
The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML.Autophagy. 2015;11(7):1114-29. doi: 10.1080/15548627.2015.1034406. Autophagy. 2015. PMID: 26029847 Free PMC article.
-
Hypoxia-Inducible Factor α Subunits Regulate Tie2-Expressing Macrophages That Influence Tumor Oxygen and Perfusion in Murine Breast Cancer.J Immunol. 2020 Oct 15;205(8):2301-2311. doi: 10.4049/jimmunol.2000185. Epub 2020 Sep 16. J Immunol. 2020. PMID: 32938724 Free PMC article.
-
Human adipose tissue-resident monocytes exhibit an endothelial-like phenotype and display angiogenic properties.Stem Cell Res Ther. 2014 Apr 14;5(2):50. doi: 10.1186/scrt438. Stem Cell Res Ther. 2014. PMID: 24731246 Free PMC article.
-
Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2.Cancer Res. 2007 Sep 15;67(18):8429-32. doi: 10.1158/0008-5472.CAN-07-1684. Cancer Res. 2007. PMID: 17875679 Review.
-
Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications.Trends Immunol. 2007 Dec;28(12):519-24. doi: 10.1016/j.it.2007.09.004. Epub 2007 Nov 5. Trends Immunol. 2007. PMID: 17981504 Review.
Cited by
-
Angiogenic miRNAs, the angiopoietin axis and related TIE2-expressing monocytes affect outcomes in cholangiocarcinoma.Oncotarget. 2018 Jul 6;9(52):29921-29933. doi: 10.18632/oncotarget.25699. eCollection 2018 Jul 6. Oncotarget. 2018. PMID: 30042823 Free PMC article.
-
The emerging roles of macrophages in cancer metastasis and response to chemotherapy.J Leukoc Biol. 2019 Aug;106(2):259-274. doi: 10.1002/JLB.MR0218-056RR. Epub 2019 Feb 5. J Leukoc Biol. 2019. PMID: 30720887 Free PMC article. Review.
-
Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy.Cancer Immunol Res. 2017 Jan;5(1):17-28. doi: 10.1158/2326-6066.CIR-16-0206. Epub 2016 Dec 21. Cancer Immunol Res. 2017. PMID: 28003187 Free PMC article.
-
Targeting Tumor-Associated Macrophages in Cancer Immunotherapy.Cancers (Basel). 2021 Oct 22;13(21):5318. doi: 10.3390/cancers13215318. Cancers (Basel). 2021. PMID: 34771482 Free PMC article. Review.
-
The impact of hypoxia on tumor-associated macrophages.J Clin Invest. 2016 Oct 3;126(10):3672-3679. doi: 10.1172/JCI84427. Epub 2016 Aug 2. J Clin Invest. 2016. PMID: 27482883 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

