Complement as a multifaceted modulator of kidney transplant injury
- PMID: 24892709
- PMCID: PMC4038571
- DOI: 10.1172/JCI72273
Complement as a multifaceted modulator of kidney transplant injury
Erratum in
-
Corrigendum. Complement as a multifaceted modulator of kidney transplant injury.J Clin Invest. 2015 Mar 2;125(3):1365. doi: 10.1172/JCI81182. Epub 2015 Mar 2. J Clin Invest. 2015. PMID: 25729858 Free PMC article. No abstract available.
Abstract
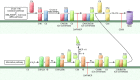
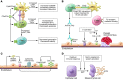
Improvements in clinical care and immunosuppressive medications have positively affected outcomes following kidney transplantation, but graft survival remains suboptimal, with half-lives of approximately 11 years. Late graft loss results from a confluence of processes initiated by ischemia-reperfusion injury and compounded by effector mechanisms of uncontrolled alloreactive T cells and anti-HLA antibodies. When combined with immunosuppressant toxicity, post-transplant diabetes and hypertension, and recurrent disease, among other factors, the result is interstitial fibrosis, tubular atrophy, and graft failure. Emerging evidence over the last decade unexpectedly identified the complement cascade as a common thread in this process. Complement activation and function affects allograft injury at essentially every step. These fundamental new insights, summarized herein, provide the foundation for testing the efficacy of various complement antagonists to improve kidney transplant function and long-term graft survival.
Figures
References
-
- Khalkhali HR, Ghafari A, Hajizadeh E, Kazemnejad A. Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction. Exp Clin Transplant. 2010;8(4):277–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

