Therapeutic translation in acute kidney injury: the epithelial/endothelial axis
- PMID: 24892710
- PMCID: PMC4089444
- DOI: 10.1172/JCI72269
Therapeutic translation in acute kidney injury: the epithelial/endothelial axis
Abstract
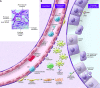
Acute kidney injury (AKI) remains a major clinical event with rising incidence, severity, and cost; it now has a morbidity and mortality exceeding acute myocardial infarction. There is also a documented conversion to and acceleration of chronic kidney disease to end-stage renal disease. The multifactorial nature of AKI etiologies and pathophysiology and the lack of diagnostic techniques have hindered translation of preclinical success. An evolving understanding of epithelial, endothelial, and inflammatory cell interactions and individualization of care will result in the eventual development of effective therapeutic strategies. This review focuses on epithelial and endothelial injury mediators, interactions, and targets for therapy.
Figures