Extracellular proteolysis of reelin by tissue plasminogen activator following synaptic potentiation
- PMID: 24892761
- PMCID: PMC4381833
- DOI: 10.1016/j.neuroscience.2014.05.046
Extracellular proteolysis of reelin by tissue plasminogen activator following synaptic potentiation
Abstract
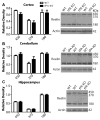
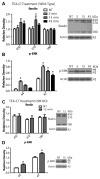
The secreted glycoprotein reelin plays an indispensable role in neuronal migration during development and in regulating adult synaptic functions. The upstream mechanisms responsible for initiating and regulating the duration and magnitude of reelin signaling are largely unknown. Here we report that reelin is cleaved between EGF-like repeats 6-7 (R6-7) by tissue plasminogen activator (tPA) under cell-free conditions. No changes were detected in the level of reelin and its fragments in the brains of tPA knockouts, implying that other unknown proteases are responsible for generating reelin fragments found constitutively in the adult brain. Induction of NMDAR-independent long-term potentiation with the potassium channel blocker tetraethylammonium chloride (TEA-Cl) led to a specific up-regulation of reelin processing at R6-7 in wild-type mice. In contrast, no changes in reelin expression and processing were observed in tPA knockouts following TEA-Cl treatment. These results demonstrate that synaptic potentiation results in tPA-dependent reelin processing and suggest that extracellular proteolysis of reelin may regulate reelin signaling in the adult brain.
Keywords: Hippocampus; Long-term potentiation; Reelin; Tetraethylammonium chloride; Tissue plasminogen activator (tPA).
Copyright © 2014. Published by Elsevier Ltd.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


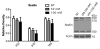
Similar articles
-
Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators.PLoS One. 2012;7(10):e47793. doi: 10.1371/journal.pone.0047793. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082219 Free PMC article.
-
Intersectin 1 is a component of the Reelin pathway to regulate neuronal migration and synaptic plasticity in the hippocampus.Proc Natl Acad Sci U S A. 2017 May 23;114(21):5533-5538. doi: 10.1073/pnas.1704447114. Epub 2017 May 8. Proc Natl Acad Sci U S A. 2017. PMID: 28484035 Free PMC article.
-
Regulation of Reelin functions by specific proteolytic processing in the brain.J Biochem. 2021 Jul 3;169(5):511-516. doi: 10.1093/jb/mvab015. J Biochem. 2021. PMID: 33566063 Review.
-
Cleavage within Reelin repeat 3 regulates the duration and range of the signaling activity of Reelin protein.J Biol Chem. 2014 May 2;289(18):12922-30. doi: 10.1074/jbc.M113.536326. Epub 2014 Mar 18. J Biol Chem. 2014. PMID: 24644294 Free PMC article.
-
Reelin and apoE actions on signal transduction, synaptic function and memory formation.Neuron Glia Biol. 2008 Aug;4(3):259-70. doi: 10.1017/S1740925X09990184. Epub 2009 Aug 13. Neuron Glia Biol. 2008. PMID: 19674510 Review.
Cited by
-
Canonical and Non-canonical Reelin Signaling.Front Cell Neurosci. 2016 Jun 30;10:166. doi: 10.3389/fncel.2016.00166. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27445693 Free PMC article. Review.
-
Reelin Signaling in Neurodevelopmental Disorders and Neurodegenerative Diseases.Brain Sci. 2023 Oct 19;13(10):1479. doi: 10.3390/brainsci13101479. Brain Sci. 2023. PMID: 37891846 Free PMC article. Review.
-
Dysfunction in the coagulation system and schizophrenia.Transl Psychiatry. 2016 Jan 5;6(1):e704. doi: 10.1038/tp.2015.204. Transl Psychiatry. 2016. PMID: 26731441 Free PMC article. Review.
-
Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome.Eur J Neurosci. 2015 May;41(10):1372-80. doi: 10.1111/ejn.12893. Epub 2015 Apr 13. Eur J Neurosci. 2015. PMID: 25864922 Free PMC article.
-
Mechanisms affecting brain remodeling in depression: do all roads lead to impaired fibrinolysis?Mol Psychiatry. 2022 Jan;27(1):525-533. doi: 10.1038/s41380-021-01264-1. Epub 2021 Aug 17. Mol Psychiatry. 2022. PMID: 34404914 Review.
References
-
- Aniksztejn L, Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. - PubMed
-
- Balmaceda V, Cuchillo-Ibanez I, Pujadas L, Garcia-Ayllon MS, Saura CA, Nimpf J, Soriano E, Saez-Valero J. ApoER2 processing by presenilin-1 modulates reelin expression. FASEB J: Off Pub Fed Am Soc Exp Biol. 2014;28:1543–1554. - PubMed
-
- Botella-Lopez A, Cuchillo-Ibanez I, Cotrufo T, Mok SS, Li QX, Barquero MS, Dierssen M, Soriano E, Saez-Valero J. Beta-amyloid controls altered reelin expression and processing in Alzheimer’s disease. Neurobiol Dis. 2010;37:682–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

