Improved testing of recent HIV-1 infections with the BioRad avidity assay compared to the limiting antigen avidity assay and BED Capture enzyme immunoassay: evaluation using reference sample panels from the German Seroconverter Cohort
- PMID: 24892795
- PMCID: PMC4043688
- DOI: 10.1371/journal.pone.0098038
Improved testing of recent HIV-1 infections with the BioRad avidity assay compared to the limiting antigen avidity assay and BED Capture enzyme immunoassay: evaluation using reference sample panels from the German Seroconverter Cohort
Abstract
Background: The variety and limitations of current laboratory methods for estimating HIV-incidence has driven attempts to improve and standardize the performance of serological 'Tests for Recent HIV-Infections' (TRI). Primary and follow-up HIV-1 positive plasma samples from individuals with well-defined dates of infection collected as part of the German Seroconverter Cohort provided specimens highly suitable for use in comparing the performance of three TRIs: the AWARE™ BED™ EIA HIV-1 Incidence test (BED-CEIA), Genetic systems HIV-1/HIV-2 Plus O EIA antibody avidity-based assay (BioRad Avidity) and Sedia™ HIV-1 LAg Avidity EIA (LAg Avidity).
Methods: The evaluation panel included 180 specimens: 44 from antiretroviral (ARV)-naïve individuals with recently acquired HIV-infection (≤ 130 days; 25 B and 19 non-B subtypes) and 136 from long-term (>12 months) infected individuals [101 ARV-naïve subtype B, 16 non-B subtypes, 14 ARV-treated individuals, 5 slow progressors (SLP)].
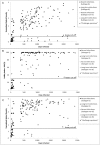
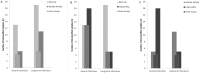
Results: For long-term infected, ARV-naïve individuals the false recent rates (FRR) of both the BioRad and LAg Avidity assays were 2% (2/101 for subtype B) and 6% (1/16 for subtype 'non-B'), while the FRR of the BED-CEIA was 7% (7/101 for subtype B) and 25% (4/16 for subtype 'non-B') (all p>0.05). Misclassification of ARV-treated individuals and SLP was rare by LAg (1/14, 0/5) and BioRad Avidity assays (2/14, 1/5) but more frequent by BED-CEIA (5/14, 3/5). Among recently-infected individuals (subtype B), 60% (15/25) were correctly classified by BED-CEIA, 88% (22/25) by BioRad Avidity and significantly fewer by LAg (48%, 12/25) compared to BioRad Avidity (p = 0.005) with a higher true-recency rate among non-B infections for all assays.
Conclusions: This study using well-characterized specimens demonstrated lower FRRs for both avidity methods than with the BED-CEIA. For recently infected individuals the BioRad Avidity assay was shown to give the most accurate results.
Conflict of interest statement
Figures

Similar articles
-
Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies.PLoS One. 2012;7(3):e33328. doi: 10.1371/journal.pone.0033328. Epub 2012 Mar 27. PLoS One. 2012. PMID: 22479384 Free PMC article.
-
Impact of HIV subtype on performance of the limiting antigen-avidity enzyme immunoassay, the bio-rad avidity assay, and the BED capture immunoassay in Rakai, Uganda.AIDS Res Hum Retroviruses. 2014 Apr;30(4):339-44. doi: 10.1089/aid.2013.0169. Epub 2013 Oct 25. AIDS Res Hum Retroviruses. 2014. PMID: 24083837 Free PMC article.
-
Estimating False-Recent Classification for the Limiting-Antigen Avidity EIA and BED-Capture Enzyme Immunoassay in Vietnam: Implications for HIV-1 Incidence Estimates.AIDS Res Hum Retroviruses. 2017 Jun;33(6):546-554. doi: 10.1089/AID.2016.0203. Epub 2017 Mar 13. AIDS Res Hum Retroviruses. 2017. PMID: 28193090 Free PMC article.
-
Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States.PLoS One. 2013 Dec 27;8(12):e82772. doi: 10.1371/journal.pone.0082772. eCollection 2013. PLoS One. 2013. PMID: 24386116 Free PMC article.
-
A systematic review of limiting antigen avidity enzyme immunoassay for detection of recent HIV-1 infection to expand supported applications.J Virus Erad. 2022 Sep 7;8(3):100085. doi: 10.1016/j.jve.2022.100085. eCollection 2022 Sep. J Virus Erad. 2022. PMID: 36124229 Free PMC article. Review.
Cited by
-
A Recent Human Immunodeficiency Virus Outbreak Among People Who Inject Drugs in Munich, Germany, Is Associated With Consumption of Synthetic Cathinones.Open Forum Infect Dis. 2020 May 27;7(6):ofaa192. doi: 10.1093/ofid/ofaa192. eCollection 2020 Jun. Open Forum Infect Dis. 2020. PMID: 32617370 Free PMC article.
-
Characterization of Humoral Immune Responses against Capsid Protein p24 and Transmembrane Glycoprotein gp41 of Human Immunodeficiency Virus Type 1 in China.PLoS One. 2016 Nov 1;11(11):e0165874. doi: 10.1371/journal.pone.0165874. eCollection 2016. PLoS One. 2016. PMID: 27802337 Free PMC article.
-
Primary HIV Drug Resistance among Recently Infected Cases of HIV in North-West India.AIDS Res Treat. 2019 Feb 27;2019:1525646. doi: 10.1155/2019/1525646. eCollection 2019. AIDS Res Treat. 2019. PMID: 30937190 Free PMC article.
-
Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review.JMIR Public Health Surveill. 2022 Mar 11;8(3):e34410. doi: 10.2196/34410. JMIR Public Health Surveill. 2022. PMID: 35275085 Free PMC article.
-
Evaluation of the limiting antigen avidity EIA (LAg) in people who inject drugs in Greece.Epidemiol Infect. 2017 Jan;145(2):401-412. doi: 10.1017/S0950268816002417. Epub 2016 Oct 26. Epidemiol Infect. 2017. PMID: 27780490 Free PMC article.
References
-
- Janssen RS, Satten GA, Stramer SL, Rawal BD, O’Brien TR, et al. (1998) New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280: 42–48. - PubMed
-
- Murphy G, Parry JV (2008) Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill 13. - PubMed
-
- Guy R, Gold J, Calleja JM, Kim AA, Parekh B, et al. (2009) Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis 9: 747–759. - PubMed
-
- Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, et al. (2010) Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24: 2763–2771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

