Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease
- PMID: 24892806
- PMCID: PMC4071383
- DOI: 10.1172/JCI71651
Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease
Abstract
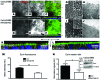
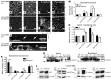
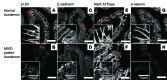
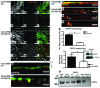
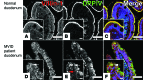
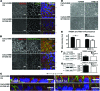
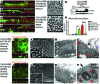
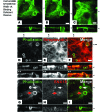
Microvillus inclusion disease (MVID) is a severe form of congenital diarrhea that arises from inactivating mutations in the gene encoding myosin Vb (MYO5B). We have examined the association of mutations in MYO5B and disruption of microvillar assembly and polarity in enterocytes. Stable MYO5B knockdown (MYO5B-KD) in CaCo2-BBE cells elicited loss of microvilli, alterations in junctional claudins, and disruption of apical and basolateral trafficking; however, no microvillus inclusions were observed in MYO5B-KD cells. Expression of WT MYO5B in MYO5B-KD cells restored microvilli; however, expression of MYO5B-P660L, a MVID-associated mutation found within Navajo populations, did not rescue the MYO5B-KD phenotype but induced formation of microvillus inclusions. Microvilli establishment required interaction between RAB8A and MYO5B, while loss of the interaction between RAB11A and MYO5B induced microvillus inclusions. Using surface biotinylation and dual immunofluorescence staining in MYO5B-KD cells expressing mutant forms of MYO5B, we observed that early microvillus inclusions were positive for the sorting marker SNX18 and derived from apical membrane internalization. In patients with MVID, MYO5B-P660L results in global changes in polarity at the villus tips that could account for deficits in apical absorption, loss of microvilli, aberrant junctions, and losses in transcellular ion transport pathways, likely leading to the MVID clinical phenotype of neonatal secretory diarrhea.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 DK70856/DK/NIDDK NIH HHS/United States
- R56 DK095811/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- R01 DK075555/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- R01 DK070856/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- R01 DK095811/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

