Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure
- PMID: 24892808
- PMCID: PMC4072023
- DOI: 10.1172/JCI73469
Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure
Abstract
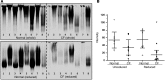
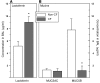
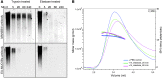
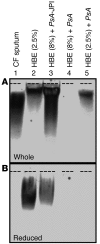
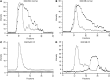
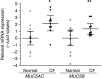
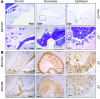
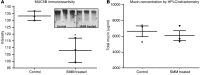
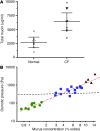
The pathogenesis of mucoinfective lung disease in cystic fibrosis (CF) patients likely involves poor mucus clearance. A recent model of mucus clearance predicts that mucus flow depends on the relative mucin concentration of the mucus layer compared with that of the periciliary layer; however, mucin concentrations have been difficult to measure in CF secretions. Here, we have shown that the concentration of mucin in CF sputum is low when measured by immunologically based techniques, and mass spectrometric analyses of CF mucins revealed mucin cleavage at antibody recognition sites. Using physical size exclusion chromatography/differential refractometry (SEC/dRI) techniques, we determined that mucin concentrations in CF secretions were higher than those in normal secretions. Measurements of partial osmotic pressures revealed that the partial osmotic pressure of CF sputum and the retained mucus in excised CF lungs were substantially greater than the partial osmotic pressure of normal secretions. Our data reveal that mucin concentration cannot be accurately measured immunologically in proteolytically active CF secretions; mucins are hyperconcentrated in CF secretions; and CF secretion osmotic pressures predict mucus layer-dependent osmotic compression of the periciliary liquid layer in CF lungs. Consequently, mucin hypersecretion likely produces mucus stasis, which contributes to key infectious and inflammatory components of CF lung disease.
Figures


References
-
- Mott LS, et al. Distribution of early structural lung changes due to cystic fibrosis detected with chest computed tomography. J Pediatr. 2013;163(1):243–248 e243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

