Micafungin in premature and non-premature infants: a systematic review of 9 clinical trials
- PMID: 24892849
- PMCID: PMC4196786
- DOI: 10.1097/INF.0000000000000434
Micafungin in premature and non-premature infants: a systematic review of 9 clinical trials
Abstract
Background: Invasive fungal infections cause excessive morbidity and mortality in premature neonates and severely ill infants.
Methods: Safety and efficacy outcomes of micafungin were compared between prematurely and non-prematurely born infants <2 years of age. Data were obtained from all completed phase I-III clinical trials with micafungin that had enrolled infants (<2 years of age) that were listed in the Astellas Clinical Study Database. Demographics, adverse events, hepatic function tests and treatment success data were extracted and validated by the Astellas biostatistical group for all micafungin-treated patients, <2 years of age, using the unique patient identifier.
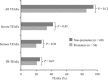
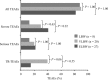
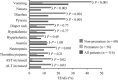
Results: One-hundred and sixteen patients included in 9 clinical trials, 48% premature [birth weight (BW) <2500 g and/or gestational age <37 weeks], 52% non-premature, received ≥ 1 dose of micafungin. Among premature patients, 14.5% were low BW (1500-2499 g), 36.4% very low BW (1000-1499 g) and 49.1% extremely low BW (<1000 g). Ninety patients (78%) completed the studies; 13 [11% (4 premature)] died. Significantly more non-premature than premature patients discontinued treatment (P = 0.003). Treatment-related adverse events were recorded in 23% of patients with no difference between groups. More extremely low BW (n = 4, 15%) and very low BW (n = 8, 40%) infants experienced treatment-related adverse events than low BW (n = 0) and there was no relation to micafungin dose or duration. For a subgroup of 30 patients with invasive candidiasis, treatment success was achieved in 73% in both premature and non-premature groups. Prophylaxis was successful in 4/5 non-premature hematopoietic stem cell transplant patients.
Conclusion: Micafungin has a safe profile in premature and non-premature infants with substantial efficacy.
Conflict of interest statement
This analysis and publication were funded by Astellas Pharma Europe Ltd. Paolo Manzoni has served as a scientific advisor and been a member on speaker bureaux for Astellas Pharma and Pfizer. Chunzhang Wu is an employee Astellas Pharma Global Development. Lorraine Tweddle is an employee Astellas Pharma Europe. Emmanuel Roilides has received research grants of significant value from Enzon, Gilead, Merck, Pfizer and Schering, has served as a scientific advisor for Astellas, Gilead, Merck, Pfizer, and Schering and been a member on speaker bureaux for Astellas, Aventis, Cephalon, Gilead, GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Schering and Wyeth.
The authors have no other funding or conflicts of interest to disclose.
Figures
Similar articles
-
[Future role of micafungin in the treatment of invasive mycoses caused by filamentous fungi].Rev Iberoam Micol. 2009 Mar 31;26(1):81-9. doi: 10.1016/S1130-1406(09)70015-4. Epub 2009 May 7. Rev Iberoam Micol. 2009. PMID: 19463284 Spanish.
-
Antifungal agents for preventing fungal infections in non-neutropenic critically ill patients.Cochrane Database Syst Rev. 2016 Jan 16;2016(1):CD004920. doi: 10.1002/14651858.CD004920.pub3. Cochrane Database Syst Rev. 2016. PMID: 26772902 Free PMC article.
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2013 Jul 02;(7):CD000361. doi: 10.1002/14651858.CD000361.pub3. PMID: 14973955 Updated.
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2001;(2):CD000361. doi: 10.1002/14651858.CD000361. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. PMID: 11405962 Updated.
-
Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002057. doi: 10.1002/14651858.CD002057.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 May 16;(5):CD002057. doi: 10.1002/14651858.CD002057.pub3. PMID: 17943765 Updated.
Cited by
-
Addressing the silent threat: managing invasive Candida infections in hospitalized newborns.Front Pediatr. 2025 Jul 17;13:1613832. doi: 10.3389/fped.2025.1613832. eCollection 2025. Front Pediatr. 2025. PMID: 40746356 Free PMC article. Review.
-
Advances in the Treatment of Mycoses in Pediatric Patients.J Fungi (Basel). 2018 Oct 11;4(4):115. doi: 10.3390/jof4040115. J Fungi (Basel). 2018. PMID: 30314389 Free PMC article. Review.
-
Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies.Pharmaceuticals (Basel). 2023 Mar 22;16(3):472. doi: 10.3390/ph16030472. Pharmaceuticals (Basel). 2023. PMID: 36986569 Free PMC article.
-
Perspectives on the Use of Echinocandins in the Neonatal Intensive Care Unit.Antibiotics (Basel). 2024 Dec 12;13(12):1209. doi: 10.3390/antibiotics13121209. Antibiotics (Basel). 2024. PMID: 39766599 Free PMC article. Review.
-
Successful therapy of Candida pulcherrima fungemia in a premature newborn with liposomal amphotericin B and micafungin.Med Mycol Case Rep. 2016 Aug 3;12:24-7. doi: 10.1016/j.mmcr.2016.08.002. eCollection 2016 Jun. Med Mycol Case Rep. 2016. PMID: 27642562 Free PMC article.
References
-
- Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F65–F69. - PubMed
-
- Roilides E. Invasive candidiasis in neonates and children. Early Hum Dev. 2011;87(Suppl 1):S75–S76. - PubMed
-
- Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–750. - PubMed
-
- Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. - PubMed
-
- Zaoutis TE, Heydon K, Localio R, et al. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

