RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer's disease
- PMID: 24892886
- PMCID: PMC4113832
- DOI: 10.1016/j.nbd.2014.05.029
RanBP9 overexpression accelerates loss of dendritic spines in a mouse model of Alzheimer's disease
Abstract
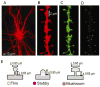
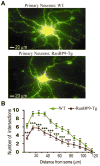
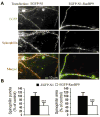
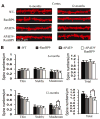
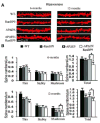
We previously demonstrated that RanBP9 overexpression increased Aβ generation and amyloid plaque burden, subsequently leading to robust reductions in the levels of several synaptic proteins as well as deficits in the learning and memory skills in a mouse model of Alzheimer's disease (AD). In the present study, we found striking reduction of spinophilin-immunoreactive puncta (52%, p<0.001) and spinophilin area (62.5%, p<0.001) in the primary cortical neurons derived from RanBP9 transgenic mice (RanBP9-Tg) compared to wild-type (WT) neurons. Similar results were confirmed in WT cortical neurons transfected with EGFP-RanBP9. At 6-months of age, the total spine density in the cortex of RanBP9 single transgenic, APΔE9 double transgenic and APΔE9/RanBP9 triple transgenic mice was similar to WT mice. However, in the hippocampus the spine density was significantly reduced (27%, p<0.05) in the triple transgenic mice compared to WT mice due to reduced number of thin spines (33%, p<0.05) and mushroom spines (22%, p<0.05). This suggests that RanBP9 overexpression in the APΔE9 mice accelerates loss of spines and that the hippocampus is more vulnerable. At 12-months of age, the cortex showed significant reductions in total spine density in the RanBP9 (22%, p<0.05), APΔE9 (19%, p<0.05) and APΔE9/RanBP9 (33%, p<0.01) mice compared to WT controls due to reductions in mushroom and thin spines. Similarly, in the hippocampus the total spine density was reduced in the RanBP9 (23%, p<0.05), APΔE9 (26%, p<0.05) and APΔE9/RanBP9 (39%, p<0.01) mice due to reductions in thin and mushroom spines. Most importantly, RanBP9 overexpression in the APΔE9 mice further exacerbated the reductions in spine density in both the cortex (14%, p<0.05) and the hippocampus (16%, p<0.05). Because dendritic spines are considered physical traces of memory, loss of spines due to RanBP9 provided the physical basis for the learning and memory deficits. Since RanBP9 protein levels are increased in AD brains, RanBP9 might play a crucial role in the loss of spines and synapses in AD.
Keywords: APΔE9 mice; Cortex; Dendritic spines; Hippocampus; RanBP9; Transgenic mice.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Ann Rev Neurosci. 2007;30:79–97. - PubMed
-
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, et al. Cortical plasticity in Alzheimer's disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

