Biochemical characterization of the phosphatase domain of the tumor suppressor PH domain leucine-rich repeat protein phosphatase
- PMID: 24892992
- PMCID: PMC4072346
- DOI: 10.1021/bi500428j
Biochemical characterization of the phosphatase domain of the tumor suppressor PH domain leucine-rich repeat protein phosphatase
Abstract
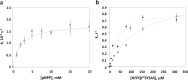
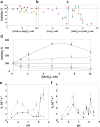
PH domain leucine-rich repeat protein phosphatase (PHLPP) directly dephosphorylates and inactivates Akt and protein kinase C and is therefore a prime target for pharmacological intervention of two key signaling pathways, the phosphatidylinositol 3-kinase and diacylglycerol signaling pathways. Here we report on the first biochemical characterization of the phosphatase domain of a PHLPP family member. The human PHLPP1 and PHLPP2 phosphatase domains were expressed and purified from bacteria or insect cells and their activities compared to that of full-length proteins immunoprecipitated from mammalian cells. Biochemical analyses reveal that the PHLPP phosphatase domain effectively dephosphorylates synthetic and peptidic substrates, that its activity is modulated by metals and lipophilic compounds, and that it has relatively high thermal stability. Mutational analysis of PHLPP2 reveals an unusual active site architecture compared to the canonical architecture of PP2C phosphatases and identifies key acidic residues (Asp 806, Glu 989, and Asp 1024) and bulky aromatic residues (Phe 783 and Phe 808) whose mutation impairs activity. Consistent with a unique active site architecture, we identify inhibitors that discriminate between PHLPP2 and PP2Cα. These data establish PHLPP as a member of the PP2C family of phosphatases with a unique active site architecture.
Figures


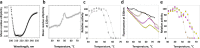


References
-
- Gao T.; Furnari F.; Newton A. C. (2005) PHLPP: A Phosphatase that Directly Dephosphorylates Akt, Promotes Apoptosis, and Suppresses Tumor Growth. Mol. Cell 18, 13–24. - PubMed
-
- Brognard J.; Sierecki E.; Gao T.; Newton A. C. (2007) PHLPP and a Second Isoform, PHLPP2, Differentially Attenuate the Amplitude of Akt Signaling by Regulating Distinct Akt Isoforms. Mol. Cell 25, 917–931. - PubMed
-
- Gao T.; Brognard J.; Newton A. C. (2008) The Phosphatase PHLPP Controls the Cellular Levels of Protein Kinase C. J. Biol. Chem. 283, 6300–6311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

