Targeting Notch1 signaling pathway positively affects the sensitivity of osteosarcoma to cisplatin by regulating the expression and/or activity of Caspase family
- PMID: 24894297
- PMCID: PMC4110525
- DOI: 10.1186/1476-4598-13-139
Targeting Notch1 signaling pathway positively affects the sensitivity of osteosarcoma to cisplatin by regulating the expression and/or activity of Caspase family
Abstract
Background: The introduction of cisplatin has improved the long-term survival rate in osteosarcoma patients. However, some patients are intrinsically resistant to cisplatin. This study reported that the activation of Notch1 is positively correlated with cisplatin sensitivity, evidenced by both clinical and in vitro data.
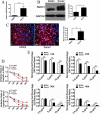
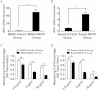
Results: In this study, a total 8 osteosarcoma specimens were enrolled and divided into two groups according to their cancer chemotherapeutic drugs sensitivity examination results. The relationship between Notch1 expression and cisplatin sensitivity of osteosarcoma patients was detected by immunohistochemistry and semi-quantitative analysis. Subsequently, two typical osteosarcoma cell lines, Saos-2 and MG63, were selected to study the changes of cisplatin sensitivity by up-regulating (NICD1 plasmid transfeciton) or decreasing (gamma-secretase complex inhibitor DAPT) the activation state of Notch1 signaling pathway. Our results showed a significant correlation between the expression of Notch1 and cisplatin sensitivity in patient specimens. In vitro, Saos-2 with higher expression of Notch1 had significantly better cisplatin sensitivity than MG63 whose Notch1 level was relatively lower. By targeting regulation in vitro, the cisplatin sensitivity of Saos-2 and MG63 had significantly increased after the activation of Notch1 signaling pathway, and vice versa. Further mechanism investigation revealed that activation/inhibition of Notch1 sensitized/desensitized cisplatin-induced apoptosis, which probably depended on the changes in the activity of Caspase family, including Caspase 3, Caspase 8 and Caspase 9 in these cells.
Conclusions: Our data clearly demonstrated that Notch1 is critical for cisplatin sensitivity in osteosarcoma. It can be used as a molecular marker and regulator for cisplatin sensitivity in osteosarcoma patients.
Figures




Similar articles
-
Doxorubicin Inhibits Proliferation of Osteosarcoma Cells Through Upregulation of the Notch Signaling Pathway.Oncol Res. 2014;22(4):185-191. doi: 10.3727/096504015X14343704124340. Oncol Res. 2014. PMID: 26351207 Free PMC article.
-
microRNA-216b enhances cisplatin-induced apoptosis in osteosarcoma MG63 and SaOS-2 cells by binding to JMJD2C and regulating the HIF1α/HES1 signaling axis.J Exp Clin Cancer Res. 2020 Sep 24;39(1):201. doi: 10.1186/s13046-020-01670-3. J Exp Clin Cancer Res. 2020. PMID: 32972441 Free PMC article.
-
Cisplatin selects for stem-like cells in osteosarcoma by activating Notch signaling.Oncotarget. 2016 May 31;7(22):33055-68. doi: 10.18632/oncotarget.8849. Oncotarget. 2016. PMID: 27102300 Free PMC article.
-
Curcumin-mediated regulation of Notch1/hairy and enhancer of split-1/survivin: molecular targeting in cholangiocarcinoma.J Surg Res. 2015 Oct;198(2):434-40. doi: 10.1016/j.jss.2015.03.029. Epub 2015 Mar 19. J Surg Res. 2015. PMID: 25890434 Review.
-
How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize.Cancer Treat Res. 2009;152:479-96. doi: 10.1007/978-1-4419-0284-9_28. Cancer Treat Res. 2009. PMID: 20213410 Review.
Cited by
-
Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3.Oncotarget. 2016 Jul 26;7(30):48296-48308. doi: 10.18632/oncotarget.10219. Oncotarget. 2016. PMID: 27340780 Free PMC article.
-
2-methylbenzoyl berbamine, a multi-targeted inhibitor, suppresses the growth of human osteosarcoma through disabling NF-κB, ERK and AKT signaling networks.Aging (Albany NY). 2020 Jul 26;12(14):15037-15049. doi: 10.18632/aging.103565. Epub 2020 Jul 26. Aging (Albany NY). 2020. PMID: 32713851 Free PMC article.
-
CEMIP Promotes Osteosarcoma Progression and Metastasis Through Activating Notch Signaling Pathway.Front Oncol. 2022 Jul 26;12:919108. doi: 10.3389/fonc.2022.919108. eCollection 2022. Front Oncol. 2022. PMID: 35957875 Free PMC article.
-
Wnt/β-Catenin Inhibition Disrupts Carboplatin Resistance in Isogenic Models of Triple-Negative Breast Cancer.Front Oncol. 2021 Jul 22;11:705384. doi: 10.3389/fonc.2021.705384. eCollection 2021. Front Oncol. 2021. PMID: 34367990 Free PMC article.
-
Zoledronic Acid Enhanced the Antitumor Effect of Cisplatin on Orthotopic Osteosarcoma by ROS-PI3K/AKT Signaling and Attenuated Osteolysis.Oxid Med Cell Longev. 2021 Mar 30;2021:6661534. doi: 10.1155/2021/6661534. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33859780 Free PMC article.
References
-
- Eyre R, Feltbower RG, Mubwandarikwa E, Eden TO, McNally RJ. Epidemiology of bone tumours in children and young adults. Pediatr Blood Cancer. 2009;53:941–952. - PubMed
-
- Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41:2836–2845. - PubMed
-
- Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, Ayala AG, Shuster JJ, Abelson HT, Simone JV, Vietti TJ. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

