Müller glial cell reprogramming and retina regeneration
- PMID: 24894585
- PMCID: PMC4249724
- DOI: 10.1038/nrn3723
Müller glial cell reprogramming and retina regeneration
Abstract
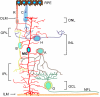
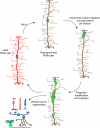
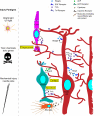
Müller glia are the major glial component of the retina. They are one of the last retinal cell types to be born during development, and they function to maintain retinal homeostasis and integrity. In mammals, Müller glia respond to retinal injury in various ways that can be either protective or detrimental to retinal function. Although these cells can be coaxed to proliferate and generate neurons under special circumstances, these responses are meagre and insufficient for repairing a damaged retina. By contrast, in teleost fish (such as zebrafish), the response of Müller glia to retinal injury involves a reprogramming event that imparts retinal stem cell characteristics and enables them to produce a proliferating population of progenitors that can regenerate all major retinal cell types and restore vision. Recent studies have revealed several important mechanisms underlying Müller glial cell reprogramming and retina regeneration in fish that may lead to new strategies for stimulating retina regeneration in mammals.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

