Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells
- PMID: 24894806
- PMCID: PMC4190909
- DOI: 10.1111/jcmm.12303
Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells
Abstract
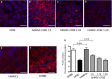
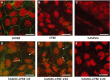
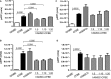
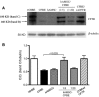
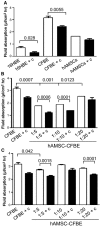
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, with most of the mortality given by the lung disease. Human amniotic mesenchymal stromal (stem) cells (hAMSCs) hold great promise for regenerative medicine in the field of lung disease; however, their potential as therapeutics for CF lung disease has not been fully explored. In the present study, hAMSCs were analysed in co-cultures on Transwell filters with CF immortalized airway epithelial cells (CFBE41o- line) at different ratios to exploit their potency to resume basic defects associated with CF. The results show that F-actin content was increased in co-cultures as compared with CF cells and actin was reorganized to form stress fibres. Confocal microscopy studies revealed that co-cultures had a tendency of increased expression of occludin and ZO-1 at the intercellular borders, paralleled by a decrease in dextran permeability, suggestive of more organized tight junctions (TJs). Spectrofluorometric analysis of CFTR function demonstrated that hAMSC-CFBE co-cultures resumed chloride transport, in line with the appearance of the mature Band C of CFTR protein by Western blotting. Moreover, hAMSC-CFBE co-cultures, at a 1:5 ratio, showed a decrease in fluid absorption, as opposed to CFBE cell monolayers that displayed a great rate of fluid resorption from the apical side. Our data show that human amniotic MSCs can be used in co-culture with CF respiratory epithelial cells to model their engraftment into the airways and have the potential to resume a tight epithelium with partial correction of the CF phenotype.
Keywords: CFTR; ENaC; actin; amniotic membrane; cell therapy; mesenchymal stromal cells; tight junctions.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45. - PubMed
-
- Matsui H, Grubb BR, Tarran R, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airway disease. Cell. 1998;95:1005–15. - PubMed
-
- Donaldson SH, Poligone EG, Stutts MJ. CFTR regulation of ENaC. Methods Mol Med. 2002;70:343–64. - PubMed
-
- Griesenbach U, Alton EWFW. Cystic fibrosis gene therapy: successes, failures and hopes for the future. Exp Rev Resp Med. 2009;3:363–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

