Nuclear adenomatous polyposis coli suppresses colitis-associated tumorigenesis in mice
- PMID: 24894865
- PMCID: PMC4123651
- DOI: 10.1093/carcin/bgu121
Nuclear adenomatous polyposis coli suppresses colitis-associated tumorigenesis in mice
Abstract
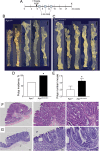
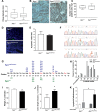
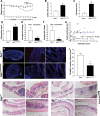
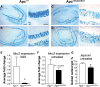
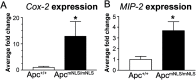
Mutation of tumor suppressor adenomatous polyposis coli (APC) initiates most colorectal cancers and chronic colitis increases risk. APC is a nucleo-cytoplasmic shuttling protein, best known for antagonizing Wnt signaling by forming a cytoplasmic complex that marks β-catenin for degradation. Using our unique mouse model with compromised nuclear Apc import (Apc(mNLS)), we show that Apc(mNLS/mNLS) mice have increased susceptibility to tumorigenesis induced with azoxymethane (AOM) and dextran sodium sulfate (DSS). The AOM-DSS-induced colon adenoma histopathology, proliferation, apoptosis, stem cell number and β-catenin and Kras mutation spectra were similar in Apc(mNLS/mNLS) and Apc(+/+) mice. However, AOM-DSS-treated Apc(mNLS/mNLS) mice showed more weight loss, more lymphoid follicles and edema, and increased colon shortening than treated Apc(+/+) mice, indicating a colitis predisposition. To test this directly, we induced acute colitis with a 7 day DSS treatment followed by 5 days of recovery. Compared with Apc(+/+) mice, DSS-treated Apc(mNLS/mNLS) mice developed more severe colitis based on clinical grade and histopathology. Apc(mNLS/mNLS) mice also had higher lymphocytic infiltration and reduced expression of stem cell markers, suggesting an increased propensity for chronic inflammation. Moreover, colons from DSS-treated Apc(mNLS/mNLS) mice showed fewer goblet cells and reduced Muc2 expression. Even in untreated Apc(mNLS/mNLS) mice, there were significantly fewer goblet cells in jejuna, and a modest decrease in colonocyte Muc2 expression compared with Apc(+/+) mice. Colonocytes from untreated Apc(mNLS/mNLS) mice also showed increased expression of inflammatory mediators cyclooxygenase-2 (Cox-2) and macrophage inflammatory protein-2 (MIP-2). These findings reveal novel functions for nuclear Apc in goblet cell differentiation and protection against inflammation-induced colon tumorigenesis.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Terzić J., et al. (2010). Inflammation and colon cancer. Gastroenterology, 138, 2101–2114.e5 - PubMed
-
- Din F.V., et al. (2010). Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut, 59, 1670–1679 - PubMed
-
- Borrello M.G., et al. (2008). Inflammation and cancer: the oncogene-driven connection. Cancer Lett., 267, 262–270 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

