The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment
- PMID: 24895166
- PMCID: PMC4714999
- DOI: 10.1007/s12307-014-0147-5
The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment
Abstract
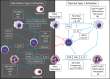
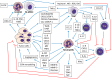
Neutrophils are myeloid cells that constitute 50-70 % of all white blood cells in the human circulation. Traditionally, neutrophils are viewed as the first line of defense against infections and as a major component of the inflammatory process. In addition, accumulating evidence suggest that neutrophils may also play a key role in multiple aspects of cancer biology. The possible involvement of neutrophils in cancer prevention and promotion was already suggested more than half a century ago, however, despite being the major component of the immune system, their contribution has often been overshadowed by other immune components such as lymphocytes and macrophages. Neutrophils seem to have conflicting functions in cancer and can be classified into anti-tumor (N1) and pro-tumor (N2) sub-populations. The aim of this review is to discuss the varying nature of neutrophil function in the cancer microenvironment with a specific emphasis on the mechanisms that regulate neutrophil mobilization, recruitment and activation.
Keywords: Chemokines; Neutrophil function; Pre-metastatic niche; Tumor Microenvironment.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

