Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice
- PMID: 24895269
- PMCID: PMC4204615
- DOI: 10.1093/gerona/glu080
Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice
Abstract
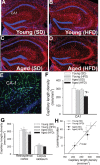
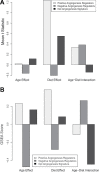
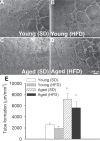
Epidemiological studies show that obesity has deleterious effects on the brain and cognitive function in the elderly population. However, the specific mechanisms through which aging and obesity interact to promote cognitive decline remain unclear. To test the hypothesis that aging exacerbates obesity-induced cerebromicrovascular impairment, we compared young (7 months) and aged (24 months) high-fat diet-fed obese C57BL/6 mice. We found that aging exacerbates the obesity-induced decline in microvascular density both in the hippocampus and in the cortex. The extent of hippocampal microvascular rarefaction and the extent of impairment of hippocampal-dependent cognitive function positively correlate. Aging exacerbates obesity-induced loss of pericyte coverage on cerebral microvessels and alters hippocampal angiogenic gene expression signature, which likely contributes to microvascular rarefaction. Aging also exacerbates obesity-induced oxidative stress and induction of NADPH oxidase and impairs cerebral blood flow responses to whisker stimulation. Collectively, obesity exerts deleterious cerebrovascular effects in aged mice, promoting cerebromicrovascular rarefaction and neurovascular uncoupling. The morphological and functional impairment of the cerebral microvasculature in association with increased blood-brain barrier disruption and neuroinflammation (Tucsek Z, Toth P, Sosnowsk D, et al. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol Biol Med Sci. 2013. In press, PMID: 24269929) likely contribute to obesity-induced cognitive decline in aging.
Keywords: Endothelial dysfunction; Learning and memory.; MCI; Vascular cognitive impairment.
© The Author 2014. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging.Age (Dordr). 2016 Aug;38(4):273-289. doi: 10.1007/s11357-016-9931-0. Epub 2016 Sep 9. Age (Dordr). 2016. PMID: 27613724 Free PMC article.
-
Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype.J Gerontol A Biol Sci Med Sci. 2018 Jun 14;73(7):853-863. doi: 10.1093/gerona/glx177. J Gerontol A Biol Sci Med Sci. 2018. PMID: 29905772 Free PMC article.
-
Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease.J Gerontol A Biol Sci Med Sci. 2014 Oct;69(10):1212-26. doi: 10.1093/gerona/glt177. Epub 2013 Nov 22. J Gerontol A Biol Sci Med Sci. 2014. PMID: 24269929 Free PMC article.
-
Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline.Exp Gerontol. 2017 Aug;94:52-58. doi: 10.1016/j.exger.2016.11.004. Epub 2016 Nov 12. Exp Gerontol. 2017. PMID: 27845201 Free PMC article. Review.
-
Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging.Am J Physiol Heart Circ Physiol. 2017 Jan 1;312(1):H1-H20. doi: 10.1152/ajpheart.00581.2016. Epub 2016 Oct 28. Am J Physiol Heart Circ Physiol. 2017. PMID: 27793855 Free PMC article. Review.
Cited by
-
"Boomerang Neuropathology" of Late-Onset Alzheimer's Disease is Shrouded in Harmful "BDDS": Breathing, Diet, Drinking, and Sleep During Aging.Neurotox Res. 2015 Jul;28(1):55-93. doi: 10.1007/s12640-015-9528-x. Epub 2015 Apr 25. Neurotox Res. 2015. PMID: 25911292 Review.
-
Endothelial Dysfunction and Impaired Neurovascular Coupling Responses Precede Cognitive Impairment in a Mouse Model of Geriatric Sepsis.Front Aging Neurosci. 2021 May 14;13:644733. doi: 10.3389/fnagi.2021.644733. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34054502 Free PMC article.
-
Pericyte loss initiates microvascular dysfunction in the development of diastolic dysfunction.Eur Heart J Open. 2023 Dec 9;4(1):oead129. doi: 10.1093/ehjopen/oead129. eCollection 2024 Jan. Eur Heart J Open. 2023. PMID: 38174347 Free PMC article.
-
A Novel Aryl Hydrocarbon Receptor Antagonist HBU651 Ameliorates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice.Int J Mol Sci. 2022 Nov 28;23(23):14871. doi: 10.3390/ijms232314871. Int J Mol Sci. 2022. PMID: 36499198 Free PMC article.
-
Neurovascular Reactivity in the Aging Mouse Brain Assessed by Laser Speckle Contrast Imaging and 2-Photon Microscopy: Quantification by an Investigator-Independent Analysis Tool.Front Neurol. 2021 Nov 11;12:745770. doi: 10.3389/fneur.2021.745770. eCollection 2021. Front Neurol. 2021. PMID: 34858312 Free PMC article.
References
-
- Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007;15:2855–2865 - PubMed
-
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116 - PubMed
-
- Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–650 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

