Inactivation of the catalytic phosphatase domain of PTPRT/RPTPρ increases social interaction in mice
- PMID: 24895325
- PMCID: PMC8970463
- DOI: 10.1002/aur.1390
Inactivation of the catalytic phosphatase domain of PTPRT/RPTPρ increases social interaction in mice
Abstract
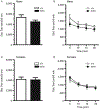
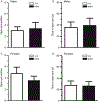
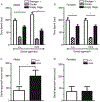
Receptor protein tyrosine phosphatase rho (RPTPρ, gene symbol PTPRT) is a transmembrane protein expressed at high levels in the developing hippocampus, olfactory bulb, cortex, and cerebellum. It has an extracellular domain that interacts with other cell adhesion molecules, and it has two intracellular phosphatase domains, one of which is catalytically active. In a recent genome-wide association study, PTPRT was identified as a potential candidate gene for autism spectrum disorder (ASD) susceptibility. Mutation of a critical aspartate to alanine (D1046A) in the PTPRT catalytic domain inactivates phosphatase function but retains substrate binding. We have generated a knockin mouse line carrying the PTPRT D1046A mutation. The D1046A mutation in homozygous knockin mice did not significantly change locomotor activities or anxiety-related behaviors. In contrast, male homozygous mice had significantly higher social approach scores than wild-type animals. Our results suggest that PTPRT phosphatase function is important in modulating neural pathways involved in mouse social behaviors relevant to the symptoms in human ASD patients.
Keywords: PTPRT; RPTPρ; animal model; social interaction.
© 2014 International Society for Autism Research, Wiley Periodicals, Inc.
Figures


Similar articles
-
Protein tyrosine phosphatase PTPRT as a regulator of synaptic formation and neuronal development.BMB Rep. 2015 May;48(5):249-55. doi: 10.5483/bmbrep.2015.48.5.037. BMB Rep. 2015. PMID: 25748173 Free PMC article. Review.
-
Synapse formation regulated by protein tyrosine phosphatase receptor T through interaction with cell adhesion molecules and Fyn.EMBO J. 2009 Nov 18;28(22):3564-78. doi: 10.1038/emboj.2009.289. Epub 2009 Oct 8. EMBO J. 2009. PMID: 19816407 Free PMC article.
-
Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT).Brain Res. 2006 Oct 20;1116(1):50-7. doi: 10.1016/j.brainres.2006.07.122. Epub 2006 Sep 14. Brain Res. 2006. PMID: 16973135
-
Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2592-7. doi: 10.1073/pnas.0914884107. Epub 2010 Jan 21. Proc Natl Acad Sci U S A. 2010. PMID: 20133777 Free PMC article.
-
Tumour suppressor function of protein tyrosine phosphatase receptor-T.Biosci Rep. 2011 Oct;31(5):303-7. doi: 10.1042/BSR20100134. Biosci Rep. 2011. PMID: 21517784 Free PMC article. Review.
Cited by
-
Hereditable variants of classical protein tyrosine phosphatase genes: Will they prove innocent or guilty?Front Cell Dev Biol. 2023 Jan 23;10:1051311. doi: 10.3389/fcell.2022.1051311. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36755664 Free PMC article. Review.
-
A novel de novo 20q13.11q13.12 microdeletion in a boy with neurodevelopmental disorders - case report.Dev Period Med. 2017;21(2):91-94. doi: 10.34763/devperiodmed.20172102.9194. Dev Period Med. 2017. PMID: 28796977 Free PMC article.
-
Protein tyrosine phosphatase PTPRT as a regulator of synaptic formation and neuronal development.BMB Rep. 2015 May;48(5):249-55. doi: 10.5483/bmbrep.2015.48.5.037. BMB Rep. 2015. PMID: 25748173 Free PMC article. Review.
References
-
- Allen-Brady K, Miller J, Matsunami N, Stevens J, Block H, et al. (2009). A high-density SNP genome-wide linkage scan in a large autism extended pedigree. Molecular Psychiatry, 14, 590–600. - PubMed
-
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. 10.1176/appi.books.9780890425596.910646. - DOI
-
- Besco JA, Hooft van Huijsduijnen R, Frostholm A, & Rotter A (2006). Intracellular substrates of brain-enriched receptor protein tyrosine phosphatase rho (RPTPrho/PTPRT). Brain Research, 1116, 50–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

