Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry
- PMID: 24895537
- PMCID: PMC4033337
- DOI: 10.1155/2014/854687
Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry
Abstract
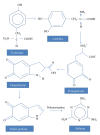
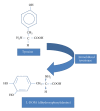
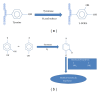
Tyrosinase is a natural enzyme and is often purified to only a low degree and it is involved in a variety of functions which mainly catalyse the o-hydroxylation of monophenols into their corresponding o-diphenols and the oxidation of o-diphenols to o-quinones using molecular oxygen, which then polymerizes to form brown or black pigments. The synthesis of o-diphenols is a potentially valuable catalytic ability and thus tyrosinase has attracted a lot of attention with respect to industrial applications. In environmental technology it is used for the detoxification of phenol-containing wastewaters and contaminated soils, as biosensors for phenol monitoring, and for the production of L-DOPA in pharmaceutical industries, and is also used in cosmetic and food industries as important catalytic enzyme. Melanin pigment synthesized by tyrosinase has found applications for protection against radiation cation exchangers, drug carriers, antioxidants, antiviral agents, or immunogen. The recombinant V. spinosum tryosinase protein can be used to produce tailor-made melanin and other polyphenolic materials using various phenols and catechols as starting materials. This review compiles the recent data on biochemical and molecular properties of microbial tyrosinases, underlining their importance in the industrial use of these enzymes. After that, their most promising applications in pharmaceutical, food processing, and environmental fields are presented.
Figures




References
-
- Robb DA. Tyrosinase. In: Lontie R, editor. Copper Proteins and Copper Enzymes. Boca Raton, Fla, USA: CRC Press; 1984. pp. 207–241.
-
- Whitaker JR. Polyphenol oxidase. In: Wong DWS, editor. Food Enzymes: Structure and Mechanism. New York, NY, USA: Chapman and Hall; 1995. pp. 271–307.
-
- Rolff M, Schottenheim J, Decker H, Tuczek F. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: molecular mechanism and comparison with the enzyme. Chemical Society Reviews. 2011;40(7):4077–4098. - PubMed
-
- dos Santos VPS, Silvaa LMC, Salgado AM, Pereira KS. Application of Agaricus bisporusextract for benzoate sodium detection based on tyrosinase inhibition for biosensor development. Chemical Engineering Transactions. 2013;32:66–70.
-
- Gradilone A, Cigna E, Agliano AM, Frati L. Tyrosinase expression as a molecular marker for investigating the presence of circulating tumor cells in melanoma patients. Current Cancer Drug Targets. 2010;10(5):529–538. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

