MicroRNA profiling as tool for in vitro developmental neurotoxicity testing: the case of sodium valproate
- PMID: 24896083
- PMCID: PMC4045889
- DOI: 10.1371/journal.pone.0098892
MicroRNA profiling as tool for in vitro developmental neurotoxicity testing: the case of sodium valproate
Abstract
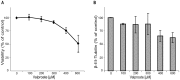
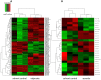
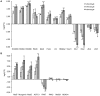
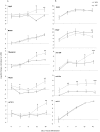
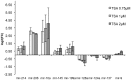
Studying chemical disturbances during neural differentiation of murine embryonic stem cells (mESCs) has been established as an alternative in vitro testing approach for the identification of developmental neurotoxicants. miRNAs represent a class of small non-coding RNA molecules involved in the regulation of neural development and ESC differentiation and specification. Thus, neural differentiation of mESCs in vitro allows investigating the role of miRNAs in chemical-mediated developmental toxicity. We analyzed changes in miRNome and transcriptome during neural differentiation of mESCs exposed to the developmental neurotoxicant sodium valproate (VPA). A total of 110 miRNAs and 377 mRNAs were identified differently expressed in neurally differentiating mESCs upon VPA treatment. Based on miRNA profiling we observed that VPA shifts the lineage specification from neural to myogenic differentiation (upregulation of muscle-abundant miRNAs, mir-206, mir-133a and mir-10a, and downregulation of neural-specific mir-124a, mir-128 and mir-137). These findings were confirmed on the mRNA level and via immunochemistry. Particularly, the expression of myogenic regulatory factors (MRFs) as well as muscle-specific genes (Actc1, calponin, myosin light chain, asporin, decorin) were found elevated, while genes involved in neurogenesis (e.g. Otx1, 2, and Zic3, 4, 5) were repressed. These results were specific for valproate treatment and--based on the following two observations--most likely due to the inhibition of histone deacetylase (HDAC) activity: (i) we did not observe any induction of muscle-specific miRNAs in neurally differentiating mESCs exposed to the unrelated developmental neurotoxicant sodium arsenite; and (ii) the expression of muscle-abundant mir-206 and mir-10a was similarly increased in cells exposed to the structurally different HDAC inhibitor trichostatin A (TSA). Based on our results we conclude that miRNA expression profiling is a suitable molecular endpoint for developmental neurotoxicity. The observed lineage shift into myogenesis, where miRNAs may play an important role, could be one of the developmental neurotoxic mechanisms of VPA.
Conflict of interest statement
Figures




References
-
- Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368: 2167–2178 doi:10.1016/S0140-6736(06)69665-7 - DOI - PubMed
-
- Landrigan PJ (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 22: 219–225 doi:10.1097/MOP.0b013e328336eb9a - DOI - PubMed
-
- Kuehn BM (2010) Increased Risk of ADHD Associated With Early Exposure to Pesticides, PCBs. JAMA 304: 27–28 doi:10.1001/jama.2010.860 - DOI - PubMed
-
- Sagiv SKS, Thurston SWS, Bellinger DCD, Tolbert PEP, Altshul LML, et al. (2010) Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171: 593–601 doi:10.1093/aje/kwp427 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

