Temperature changes in brown adipocytes detected with a bimaterial microcantilever
- PMID: 24896125
- PMCID: PMC4052269
- DOI: 10.1016/j.bpj.2014.04.044
Temperature changes in brown adipocytes detected with a bimaterial microcantilever
Abstract
Mammalian cells must produce heat to maintain body temperature and support other biological activities. Methods to measure a cell's thermogenic ability by inserting a thermometer into the cell or measuring the rate of oxygen consumption in a closed vessel can disturb its natural state. Here, we developed a noninvasive system for measuring a cell's heat production with a bimaterial microcantilever. This method is suitable for investigating the heat-generating properties of cells in their native state, because changes in cell temperature can be measured from the bending of the microcantilever, without damaging the cell and restricting its supply of dissolved oxygen. Thus, we were able to measure increases in cell temperature of <1 K in a small number of murine brown adipocytes (n = 4-7 cells) stimulated with norepinephrine, and observed a slow increase in temperature over several hours. This long-term heat production suggests that, in addition to converting fatty acids into heat energy, brown adipocytes may also adjust protein expression to raise their own temperature, to generate more heat. We expect this bimaterial microcantilever system to prove useful for determining a cell's state by measuring thermal characteristics.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
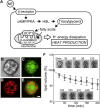


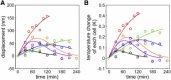
References
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. - PubMed
-
- Nedergaard J., Cannon B., Lindberg O. Microcalorimetry of isolated mammalian cells. Nature. 1977;267:518–520. - PubMed
-
- Hansen E.S., Knudsen J. Parallel measurements of heat production and thermogenin content in brown fat cells during cold acclimation of rats. Biosci. Rep. 1986;6:31–38. - PubMed
-
- Tanaka E., Yamakawa A., Nakazawa H. Regulation of heat production of brown adipocytes via typical and atypical β-adrenoceptors in the rat. Jpn. J. Physiol. 1995;45:1043–1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

