Hyperhomocysteinemia promotes vascular remodeling in vein graph in mice
- PMID: 24896329
- PMCID: PMC4331018
- DOI: 10.2741/4260
Hyperhomocysteinemia promotes vascular remodeling in vein graph in mice
Abstract
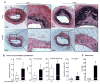
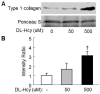
This study investigated the role and mechanism of Hyperhomocysteinemia (HHcy) on vascular remodeling in mice. We assessed the effect of HHcy on vascular remodeling using a carotid arterial vein patch model in mice with the gene deletion of cystathionine-beta-synthase (Cbs). Vein grafts were harvested 4 weeks after surgery. Cross sections were analyzed using Verhoeff-van Gieson staining, Masson`s Trichrome staining, and immunostaining for morphological analysis and protein level assessment. The effect of Hcy on collagen secretion was examined in cultured rat aortic smooth muscle cells (RASMC). We found that Cbs-/- mice with severe HHcy exhibited thicker neointima and a higher percentage of luminal narrowing in vein grafts. In addition, severe HHcy increased elastin and collagen deposition in the neointima. Further, severe HHcy increases CD45 positive cells and proliferative cells in vein grafts. Finally, Hcy increases collagen secretion in RASMC. These results demonstrate that HHcy increases neointima formation, elastin and collagen deposition following a carotid arterial vein patch. The capacity of Hcy to promote vascular fibrosis and inflammation may contribute to the development of vascular remodeling.
Figures



Similar articles
-
Hyperhomocysteinemia inhibits post-injury reendothelialization in mice.Cardiovasc Res. 2006 Jan;69(1):253-62. doi: 10.1016/j.cardiores.2005.08.016. Epub 2005 Oct 13. Cardiovasc Res. 2006. PMID: 16226235 Free PMC article.
-
Mesenteric vascular remodeling in hyperhomocysteinemia.Mol Cell Biochem. 2011 Feb;348(1-2):99-108. doi: 10.1007/s11010-010-0643-y. Epub 2010 Nov 13. Mol Cell Biochem. 2011. PMID: 21076854
-
Hyperhomocysteinemia suppresses bone marrow CD34+/VEGF receptor 2+ cells and inhibits progenitor cell mobilization and homing to injured vasculature-a role of β1-integrin in progenitor cell migration and adhesion.FASEB J. 2015 Jul;29(7):3085-99. doi: 10.1096/fj.14-267989. Epub 2015 Apr 8. FASEB J. 2015. PMID: 25854700 Free PMC article.
-
Mechanisms of cardiovascular remodeling in hyperhomocysteinemia.Antioxid Redox Signal. 2011 Oct 1;15(7):1927-43. doi: 10.1089/ars.2010.3721. Epub 2011 Apr 21. Antioxid Redox Signal. 2011. PMID: 21126196 Free PMC article. Review.
-
Homocysteine to hydrogen sulfide or hypertension.Cell Biochem Biophys. 2010 Jul;57(2-3):49-58. doi: 10.1007/s12013-010-9079-y. Cell Biochem Biophys. 2010. PMID: 20387006 Free PMC article. Review.
Cited by
-
Vein graft disease in a knockout mouse model of hyperhomocysteinaemia.Int J Exp Pathol. 2016 Dec;97(6):447-456. doi: 10.1111/iep.12215. Epub 2016 Dec 22. Int J Exp Pathol. 2016. PMID: 28004436 Free PMC article.
-
Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration.J Mol Biol. 2017 Feb 17;429(4):543-561. doi: 10.1016/j.jmb.2016.12.015. Epub 2016 Dec 21. J Mol Biol. 2017. PMID: 28013031 Free PMC article. Review.
-
Circulating homocysteine and folate concentrations and risk of type 2 diabetes: A retrospective observational study in Chinese adults and a Mendelian randomization analysis.Front Cardiovasc Med. 2022 Nov 14;9:978998. doi: 10.3389/fcvm.2022.978998. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36483625 Free PMC article.
-
Plasma Levels of Homocysteine is Associated with Liver Fibrosis in Health Check-Up Population.Int J Gen Med. 2021 Sep 3;14:5175-5181. doi: 10.2147/IJGM.S329863. eCollection 2021. Int J Gen Med. 2021. PMID: 34512000 Free PMC article.
References
-
- Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324(17):1149–1155. - PubMed
-
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–236. - PubMed
-
- Cheng Z, Jiang X, Kruger WD, Pratico D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang X, Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118(7):1998–2006. - PMC - PubMed
-
- Woo CW, Siow YL, Pierce GN, Choy PC, Minuk GY, Mymin D, OK Hyperhomocysteinemia induces hepatic cholesterol biosynthesis and lipid accumulation via activation of transcription factors. Am J Physiol Endocrinol Metab. 2005;288(5):E1002–1010. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- HL82774/HL/NHLBI NIH HHS/United States
- R01 HL108910/HL/NHLBI NIH HHS/United States
- R01 HL077288/HL/NHLBI NIH HHS/United States
- HL108910/HL/NHLBI NIH HHS/United States
- R01 HL117654/HL/NHLBI NIH HHS/United States
- HL67033/HL/NHLBI NIH HHS/United States
- R01 HL082774/HL/NHLBI NIH HHS/United States
- R01 HL110764/HL/NHLBI NIH HHS/United States
- HL110764/HL/NHLBI NIH HHS/United States
- HL116917/HL/NHLBI NIH HHS/United States
- R01 HL094451/HL/NHLBI NIH HHS/United States
- HL9445/HL/NHLBI NIH HHS/United States
- F32 HL009445/HL/NHLBI NIH HHS/United States
- HL77288/HL/NHLBI NIH HHS/United States
- HL117654/HL/NHLBI NIH HHS/United States
- R01 HL067033/HL/NHLBI NIH HHS/United States
- R01 HL116917/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

