The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells
- PMID: 24896565
- PMCID: PMC4126086
- DOI: 10.1038/jcbfm.2014.100
The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells
Abstract
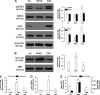
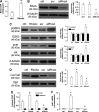
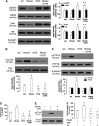
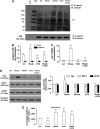
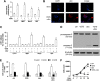
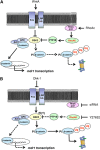
In this work, we investigate if and how transducers of the 'canonical' Wnt pathway, i.e., Wnt/glycogen synthase kinase 3 (GSK3)/β-catenin, and transducers of the 'non-canonical' Wnt pathway, i.e., Wnt/RhoA/RhoA kinase (RhoAK), cooperate to control the expression of P-glycoprotein (Pgp) in blood-brain barrier (BBB) cells. By analyzing human primary brain microvascular endothelial cells constitutively activated for RhoA, silenced for RhoA or treated with the RhoAK inhibitor Y27632, we found that RhoAK phosphorylated and activated the protein tyrosine phosphatase 1B (PTP1B), which dephosphorylated tyrosine 216 of GSK3, decreasing the GSK3-mediated inhibition of β-catenin. By contrast, the inhibition of RhoA/RhoAK axis prevented the activation of PTP1B, enhanced the GSK3-induced phosphorylation and ubiquitination of β-catenin, and reduced the β-catenin-driven transcription of Pgp. The RhoAK inhibition increased the delivery of Pgp substrates like doxorubicin across the BBB and improved the doxorubicin efficacy against glioblastoma cells co-cultured under a BBB monolayer. Our data demonstrate that in human BBB cells the expression of Pgp is controlled by a cross-talk between canonical and non-canonical Wnt pathways. The disruption of this cross-talk, e.g., by inhibiting RhoAK, downregulates Pgp and increases the delivery of Pgp substrates across the BBB.
Figures
Comment in
-
Frizzled fissure to improve central nervous system drug delivery?J Cereb Blood Flow Metab. 2014 Aug;34(8):1257. doi: 10.1038/jcbfm.2014.98. Epub 2014 Jun 4. J Cereb Blood Flow Metab. 2014. PMID: 24896568 Free PMC article.
Similar articles
-
Frizzled fissure to improve central nervous system drug delivery?J Cereb Blood Flow Metab. 2014 Aug;34(8):1257. doi: 10.1038/jcbfm.2014.98. Epub 2014 Jun 4. J Cereb Blood Flow Metab. 2014. PMID: 24896568 Free PMC article.
-
Wnt3A Induces GSK-3β Phosphorylation and β-Catenin Accumulation Through RhoA/ROCK.J Cell Physiol. 2017 May;232(5):1104-1113. doi: 10.1002/jcp.25572. Epub 2016 Nov 20. J Cell Physiol. 2017. PMID: 27575935
-
Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/β-catenin pathway.Neuro Oncol. 2013 Nov;15(11):1502-17. doi: 10.1093/neuonc/not104. Epub 2013 Jul 28. Neuro Oncol. 2013. PMID: 23897632 Free PMC article.
-
Intracellular Signals Activated by Canonical Wnt Ligands Independent of GSK3 Inhibition and β-Catenin Stabilization.Cells. 2019 Sep 25;8(10):1148. doi: 10.3390/cells8101148. Cells. 2019. PMID: 31557964 Free PMC article. Review.
-
Selective treatment of mixed-lineage leukemia leukemic stem cells through targeting glycogen synthase kinase 3 and the canonical Wnt/β-catenin pathway.Curr Opin Hematol. 2012 Jul;19(4):280-6. doi: 10.1097/MOH.0b013e3283545615. Curr Opin Hematol. 2012. PMID: 22525581 Review.
Cited by
-
New Tetrahydroisoquinoline Derivatives Overcome Pgp Activity in Brain-Blood Barrier and Glioblastoma Multiforme in Vitro.Molecules. 2018 Jun 9;23(6):1401. doi: 10.3390/molecules23061401. Molecules. 2018. PMID: 29890725 Free PMC article.
-
Suppressing Wnt signaling of the blood‒tumor barrier to intensify drug delivery and inhibit lipogenesis of brain metastases.Acta Pharm Sin B. 2024 Jun;14(6):2716-2731. doi: 10.1016/j.apsb.2024.03.024. Epub 2024 Mar 21. Acta Pharm Sin B. 2024. PMID: 38828148 Free PMC article.
-
Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells.Sci Rep. 2019 Dec 23;9(1):19718. doi: 10.1038/s41598-019-56075-w. Sci Rep. 2019. PMID: 31873116 Free PMC article.
-
Lithium attenuates blood-brain barrier damage and brain edema following intracerebral hemorrhage via an endothelial Wnt/β-catenin signaling-dependent mechanism in mice.CNS Neurosci Ther. 2022 Jun;28(6):862-872. doi: 10.1111/cns.13832. Epub 2022 Mar 28. CNS Neurosci Ther. 2022. PMID: 35343071 Free PMC article.
-
RHOA in Gastric Cancer: Functional Roles and Therapeutic Potential.Front Genet. 2019 May 15;10:438. doi: 10.3389/fgene.2019.00438. eCollection 2019. Front Genet. 2019. PMID: 31156701 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

