Highly energized inhibitory interneurons are a central element for information processing in cortical networks
- PMID: 24896567
- PMCID: PMC4126088
- DOI: 10.1038/jcbfm.2014.104
Highly energized inhibitory interneurons are a central element for information processing in cortical networks
Abstract
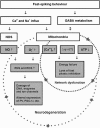
Gamma oscillations (∼30 to 100 Hz) provide a fundamental mechanism of information processing during sensory perception, motor behavior, and memory formation by coordination of neuronal activity in networks of the hippocampus and neocortex. We review the cellular mechanisms of gamma oscillations about the underlying neuroenergetics, i.e., high oxygen consumption rate and exquisite sensitivity to metabolic stress during hypoxia or poisoning of mitochondrial oxidative phosphorylation. Gamma oscillations emerge from the precise synaptic interactions of excitatory pyramidal cells and inhibitory GABAergic interneurons. In particular, specialized interneurons such as parvalbumin-positive basket cells generate action potentials at high frequency ('fast-spiking') and synchronize the activity of numerous pyramidal cells by rhythmic inhibition ('clockwork'). As prerequisites, fast-spiking interneurons have unique electrophysiological properties and particularly high energy utilization, which is reflected in the ultrastructure by enrichment with mitochondria and cytochrome c oxidase, most likely needed for extensive membrane ion transport and γ-aminobutyric acid metabolism. This supports the hypothesis that highly energized fast-spiking interneurons are a central element for cortical information processing and may be critical for cognitive decline when energy supply becomes limited ('interneuron energy hypothesis'). As a clinical perspective, we discuss the functional consequences of metabolic and oxidative stress in fast-spiking interneurons in aging, ischemia, Alzheimer's disease, and schizophrenia.
Figures


References
-
- Siesjö BK.(ed). Brain Energy Metabolism John Wiley & Sons: New York, USA; 1978
-
- Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. - PubMed
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

