Glutathione metabolism in Candida albicans resistant strains to fluconazole and micafungin
- PMID: 24896636
- PMCID: PMC4045664
- DOI: 10.1371/journal.pone.0098387
Glutathione metabolism in Candida albicans resistant strains to fluconazole and micafungin
Abstract
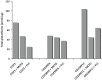
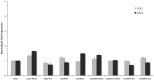
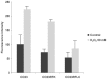
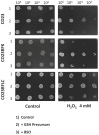
Currently available therapies for candidiasis are based on antifungal drugs belonging to azole and echinocandin families that interfere with different aspects of fungal metabolism. These drugs, beyond their specific effects, elicit also a cellular stress including an unbalance of redox state that is counteracted not only utilizing antioxidant species but also increasing the outcome export by transporters to detoxify the internal environment. These cellular actions are both based on the cytosolic concentration of reduced glutathione (GSH). In this paper we investigated the effects of two antifungal drugs fluconazole and micafungin on the redox state of the cell in C. albicans to understand if the resistance to these drugs is accompanied by variation of glutathione metabolism. Analyses of resistant strains showed a marked difference in glutathione contents in strains resistant to fluconazole (CO23RFLC) or micafungin (CO23RFK). In CO23RFLC, the total amount of glutathione was more than doubled with respect to CO23RFK thanks to the increased activity of γ-glutamilcysteine synthetase, the key enzyme involved in GSH synthesis. We demonstrated that the GSH increase in CO23RFLC conferred to this strain a clear advantage in counteracting oxidative toxic agents while assignment of other roles, such as a more efficient elimination of the drug from the cell, should be considered more speculative. As far as MCFG resistance is concerned, from our data a role of glutathione metabolism in supporting this condition is not evident. Overall our data indicate that glutathione metabolism is differently affected in the two resistant strains and that glutathione system may play an important role in the global organization of C.albicans cells for resistance to fluconazole. Such scenario may pave the way to hypothesize the use of oxidant drugs or inhibitors able to deplete reduced glutathione level as a novel approach, for counteracting the resistance to this specific antifungal drug.
Conflict of interest statement
Figures
Similar articles
-
Characterization of biofilms in drug-sensitive and drug-resistant strains of Candida albicans.J Chemother. 2013 Apr;25(2):87-95. doi: 10.1179/1973947812Y.0000000047. J Chemother. 2013. PMID: 23684356
-
The efficiency of the benzothiazole APB, the echinocandin micafungin, and amphotericin B in fluconazole-resistant Candida albicans and Candida dubliniensis.Pharmazie. 2004 Jul;59(7):573-4. Pharmazie. 2004. PMID: 15296100
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp.J Clin Microbiol. 2006 Feb;44(2):324-6. doi: 10.1128/JCM.44.2.324-326.2006. J Clin Microbiol. 2006. PMID: 16455878 Free PMC article.
-
Micafungin: a review of its use in the prophylaxis and treatment of invasive Candida infections.Drugs. 2012 Nov 12;72(16):2141-65. doi: 10.2165/11209970-000000000-00000. Drugs. 2012. PMID: 23083111 Review.
Cited by
-
Integrative functional analysis uncovers metabolic differences between Candida species.Commun Biol. 2022 Sep 26;5(1):1013. doi: 10.1038/s42003-022-03955-z. Commun Biol. 2022. PMID: 36163459 Free PMC article.
-
Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells.Biochim Biophys Acta Gen Subj. 2017 Jan;1861(1 Pt A):3002-3010. doi: 10.1016/j.bbagen.2016.09.029. Epub 2016 Oct 3. Biochim Biophys Acta Gen Subj. 2017. PMID: 27712973 Free PMC article.
-
Antifungal Activity of Human Cathelicidin LL-37, a Membrane Disrupting Peptide, by Triggering Oxidative Stress and Cell Cycle Arrest in Candida auris.J Fungi (Basel). 2022 Feb 20;8(2):204. doi: 10.3390/jof8020204. J Fungi (Basel). 2022. PMID: 35205958 Free PMC article.
-
The Transcription Factor Sfp1 Regulates the Oxidative Stress Response in Candida albicans.Microorganisms. 2019 May 14;7(5):131. doi: 10.3390/microorganisms7050131. Microorganisms. 2019. PMID: 31091716 Free PMC article.
-
Anticandidal Activity of a Siderophore from Marine Endophyte Pseudomonas aeruginosa Mgrv7.Antibiotics (Basel). 2024 Apr 10;13(4):347. doi: 10.3390/antibiotics13040347. Antibiotics (Basel). 2024. PMID: 38667023 Free PMC article.
References
-
- Calderone RA (2002) Candida and Candidiasis. ASM Press; Washington, DC.
-
- Odds FC, AJP Brown, NAR Gow (2003) Antifungal agents: mechanisms of action. Trends in microbiology 11: 272–79. - PubMed
-
- Kim J, Sudbery P (2011) Candida albicans, a major human fungal pathogen. J Microbiol 49: 171–7. - PubMed
-
- Perlroth J, Choi B, Spellberg B (2007) Nosocomial fungal infection: epidemiology, diagnosis and treatment. Med Mycol 45(4): 321–46. - PubMed
-
- Akins RA (2005) An update on antifungal targets and mechanism of resistance in Candida albicans . Med Mycol 43(4): 285–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

