Manganese-induced atypical parkinsonism is associated with altered Basal Ganglia activity and changes in tissue levels of monoamines in the rat
- PMID: 24896650
- PMCID: PMC4045849
- DOI: 10.1371/journal.pone.0098952
Manganese-induced atypical parkinsonism is associated with altered Basal Ganglia activity and changes in tissue levels of monoamines in the rat
Abstract
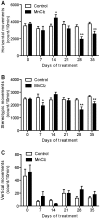
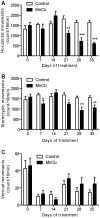
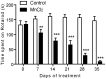
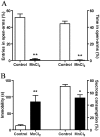
Manganese neurotoxicity is associated with motor and cognitive disturbances known as Manganism. However, the mechanisms underlying these deficits remain unknown. Here we investigated the effects of manganese intoxication on motor and non-motor parkinsonian-like deficits such as locomotor activity, motor coordination, anxiety and "depressive-like" behaviors. Then, we studied the impact of this intoxication on the neuronal activity, the globus pallidus (GP) and subthalamic nucleus (STN). At the end of experiments, post-mortem tissue level of the three monoamines (dopamine, norepinephrine and serotonin) has been determined. The experiments were carried out in adult Sprague-Dawley rats, daily treated with MnCl2 (10 mg/kg/, i.p.) for 5 weeks. We show that manganese progressively reduced locomotor activity as well as motor coordination in parallel with the manifestation of anxiety and "depressive-like" behaviors. Electrophysiological results show that, while majority of GP and STN neurons discharged regularly in controls, manganese increased the number of GP and STN neurons discharging irregularly and/or with bursts. Biochemical results show that manganese significantly decreased tissue levels of norepinephrine and serotonin with increased metabolism of dopamine in the striatum. Our data provide evidence that manganese intoxication is associated with impaired neurotransmission of monoaminergic systems, which is at the origin of changes in basal ganglia neuronal activity and the manifestation of motor and non-motor deficits similar to those observed in atypical Parkinsonism.
Conflict of interest statement
Figures




Similar articles
-
Lead-Induced Atypical Parkinsonism in Rats: Behavioral, Electrophysiological, and Neurochemical Evidence for a Role of Noradrenaline Depletion.Front Neurosci. 2018 Mar 19;12:173. doi: 10.3389/fnins.2018.00173. eCollection 2018. Front Neurosci. 2018. PMID: 29615861 Free PMC article.
-
Lead intoxication induces noradrenaline depletion, motor nonmotor disabilities, and changes in the firing pattern of subthalamic nucleus neurons.Neuroscience. 2012 May 17;210:375-83. doi: 10.1016/j.neuroscience.2012.02.026. Epub 2012 Feb 21. Neuroscience. 2012. PMID: 22421103
-
The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat.Neuroscience. 2015 Dec 17;311:118-29. doi: 10.1016/j.neuroscience.2015.10.021. Epub 2015 Oct 20. Neuroscience. 2015. PMID: 26477982
-
Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease.Mov Disord. 2008;23 Suppl 3:S548-59. doi: 10.1002/mds.22062. Mov Disord. 2008. PMID: 18781672 Review.
-
The neuropathology of manganese-induced Parkinsonism.J Neuropathol Exp Neurol. 2007 Aug;66(8):675-82. doi: 10.1097/nen.0b013e31812503cf. J Neuropathol Exp Neurol. 2007. PMID: 17882011 Review.
Cited by
-
Progress and trends of research on mineral elements for depression.Heliyon. 2024 Jul 31;10(15):e35469. doi: 10.1016/j.heliyon.2024.e35469. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170573 Free PMC article.
-
Neuroprotective Effects of Some Nutraceuticals against Manganese-Induced Parkinson's Disease in Rats: Possible Modulatory Effects on TLR4/NLRP3/NF-κB, GSK-3β, Nrf2/HO-1, and Apoptotic Pathways.Pharmaceuticals (Basel). 2022 Dec 14;15(12):1554. doi: 10.3390/ph15121554. Pharmaceuticals (Basel). 2022. PMID: 36559006 Free PMC article.
-
Parkinson's Disease and the Metal-Microbiome-Gut-Brain Axis: A Systems Toxicology Approach.Antioxidants (Basel). 2021 Dec 28;11(1):71. doi: 10.3390/antiox11010071. Antioxidants (Basel). 2021. PMID: 35052575 Free PMC article. Review.
-
Manganese-induced Neurotoxicity: From C. elegans to Humans.Toxicol Res (Camb). 2015 Mar 1;4(2):191-202. doi: 10.1039/C4TX00127C. Toxicol Res (Camb). 2015. PMID: 25893090 Free PMC article.
-
Copper, Iron, and Manganese Toxicity in Neuropsychiatric Conditions.Int J Mol Sci. 2021 Jul 22;22(15):7820. doi: 10.3390/ijms22157820. Int J Mol Sci. 2021. PMID: 34360586 Free PMC article. Review.
References
-
- Takeda A (2003) Manganese action in brain function. Brain Research Reviews 41: 79–87. - PubMed
-
- Aschner M, Erikson KM, Dorman DC (2005) Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol 35: 1–32. - PubMed
-
- Dobson AW, Erikson KM, Aschner M (2004) Manganese Neurotoxicity. Annals of the New York Academy of Sciences 1012: 115–128. - PubMed
-
- Perl DP, Olanow CW (2007) The Neuropathology of Manganese-Induced Parkinsonism. Journal of Neuropathology & Experimental Neurology 66: 675–682 610.1097/nen.1090b1013e31812503cf. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

