Transient transfection of CHO cells using linear polyethylenimine is a simple and effective means of producing rainbow trout recombinant IFN-γ protein
- PMID: 24897997
- PMCID: PMC4628921
- DOI: 10.1007/s10616-014-9737-9
Transient transfection of CHO cells using linear polyethylenimine is a simple and effective means of producing rainbow trout recombinant IFN-γ protein
Abstract
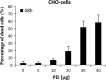
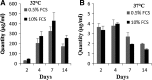
A practical method was developed for the transient transfection of Chinese hamster ovary (CHO) cells with 25 kDa linear polyethylenimine (PEI) then optimal culture conditions determined for the production of rainbow trout (Oncorhynchus mykiss) IFN-γ recombinant protein. We found that culture temperature had a significant impact upon recombinant protein yield, with best results being obtained at 32 °C. However the amount of serum added to the culture medium had no effect upon recombinant IFN-γ (rIFN-γ) production. In this study maximal rIFN-γ yields and minimal PEI toxicity were achieved using a DNA/PEI ratio of 1:8, where the amount of PEI did not exceed 10 µg per 5 ml of RPMI1640 culture medium, with cells subsequently cultured at 32 °C for 7 days. Thus, linear PEI is a technically simple and cost-efficient method for the transient transfection of CHO cells and is compatible with serum-free operations.
Keywords: Chinese hamster ovary cells; Linear polyethylenimine; Rainbow trout IFN-γ; Recombinant protein; Transient transfection.
Figures



Similar articles
-
Iron (III) citrate inhibits polyethylenimine-mediated transient transfection of Chinese hamster ovary cells in serum-free medium.Cytotechnology. 2009 Jul;60(1-3):1. doi: 10.1007/s10616-009-9198-8. Epub 2009 Jun 26. Cytotechnology. 2009. PMID: 19557539 Free PMC article.
-
Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/DNA complexes in combination with microtubule disrupting anti-mitotic agents.Biotechnol Bioeng. 2004 Dec 20;88(6):707-21. doi: 10.1002/bit.20265. Biotechnol Bioeng. 2004. PMID: 15532040
-
Control of culture environment for improved polyethylenimine-mediated transient production of recombinant monoclonal antibodies by CHO cells.Biotechnol Prog. 2006 May-Jun;22(3):753-62. doi: 10.1021/bp050339v. Biotechnol Prog. 2006. PMID: 16739959
-
Serum-free large-scale transient transfection of CHO cells.Biotechnol Bioeng. 2004 Aug 20;87(4):537-45. doi: 10.1002/bit.20161. Biotechnol Bioeng. 2004. PMID: 15286991
-
Enhancing recombinant antibody yield in Chinese hamster ovary cells.Tzu Chi Med J. 2024 May 24;36(3):240-250. doi: 10.4103/tcmj.tcmj_315_23. eCollection 2024 Jul-Sep. Tzu Chi Med J. 2024. PMID: 38993821 Free PMC article. Review.
Cited by
-
Preclinical immunological evaluation of an intradermal heterologous vaccine against SARS-CoV-2 variants.Emerg Microbes Infect. 2022 Dec;11(1):212-226. doi: 10.1080/22221751.2021.2021807. Emerg Microbes Infect. 2022. PMID: 34931939 Free PMC article.
-
A transposon-based transient transfection system in CHO-K1 cells enables quality prediction of stable cell line proteins.Bioprocess Biosyst Eng. 2025 Sep;48(9):1587-1597. doi: 10.1007/s00449-025-03198-2. Epub 2025 Jul 12. Bioprocess Biosyst Eng. 2025. PMID: 40650747
-
Long-Term Cross Immune Response in Mice following Heterologous Prime-Boost COVID-19 Vaccination with Full-Length Spike mRNA and Recombinant S1 Protein.Vaccines (Basel). 2023 May 9;11(5):963. doi: 10.3390/vaccines11050963. Vaccines (Basel). 2023. PMID: 37243067 Free PMC article.
-
PM2.5 induces cell cycle arrest through regulating mTOR/P70S6K1 signaling pathway.Exp Ther Med. 2019 Jun;17(6):4371-4378. doi: 10.3892/etm.2019.7466. Epub 2019 Apr 3. Exp Ther Med. 2019. PMID: 31086573 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

