Histone deacetylase inhibitors and cell death
- PMID: 24898083
- PMCID: PMC4414051
- DOI: 10.1007/s00018-014-1656-6
Histone deacetylase inhibitors and cell death
Abstract
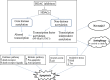
Histone deacetylases (HDACs) are a vast family of enzymes involved in chromatin remodeling and have crucial roles in numerous biological processes, largely through their repressive influence on transcription. In addition to modifying histones, HDACs also target many other non-histone protein substrates to regulate gene expression. Recently, HDACs have gained growing attention as HDAC-inhibiting compounds are being developed as promising cancer therapeutics. Histone deacetylase inhibitors (HDACi) have been shown to induce differentiation, cell cycle arrest, apoptosis, autophagy and necrosis in a variety of transformed cell lines. In this review, we mainly discuss how HDACi may elicit a therapeutic response to human cancers through different cell death pathways, in particular, apoptosis and autophagy.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

