Failure to address potential bias in non-randomised controlled clinical trials may cause lack of evidence on patient-reported outcomes: a method study
- PMID: 24898087
- PMCID: PMC4054649
- DOI: 10.1136/bmjopen-2013-004720
Failure to address potential bias in non-randomised controlled clinical trials may cause lack of evidence on patient-reported outcomes: a method study
Abstract
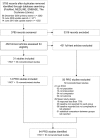
Objectives: We conducted a workup of a previously published systematic review and aimed to analyse why most of the identified non-randomised controlled clinical trials with patient-reported outcomes did not match a set of basic quality criteria.
Setting: There were no limits on the level of care and the geographical location.
Participants: The review evaluated permanent interstitial low-dose rate brachytherapy in patients with localised prostate cancer and compared that intervention with alternative procedures such as external beam radiotherapy, radical prostatectomy and no primary therapy.
Primary outcome measure: Fulfilment of basic inclusion criteria according to a Participants, Interventions, Comparisons, Outcomes (PICO) framework and accomplishment of requirements to contain superimposed risk of bias.
Results: We found that 21 of 50 excluded non-randomised controlled trials did not meet the PICO inclusion criteria. The remaining 29 studies showed a lack in the quality of reporting. The resulting flaws included attrition bias due to loss of follow-up, lack of reporting baseline data, potential confounding due to unadjusted data and lack of statistical comparison between groups.
Conclusions: With respect to the reporting of patient-reported outcomes, active efforts are required to improve the quality of reporting in non-randomised controlled trials concerning permanent interstitial low-dose rate brachytherapy in patients with localised prostate cancer.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Peinemann F, Grouven U, Bartel C, et al. Permanent interstitial low-dose rate brachytherapy for patients with localized prostate cancer—a systematic review of randomized and non-randomized controlled clinical trials. Eur Urol 2011;60:881–93 - PubMed
-
- Patrick D, Guyatt GH, Acquadro C. Chapter 17: Patient-reported outcomes. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions Version 510 [updated March 2011]. Chichester: The Cochrane Collaboration, 2011. www.cochrane-handbook.org
-
- Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814–22 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical