Use of tenofovir disoproxil fumarate in highly viremic, hepatitis B mono-infected pregnant women
- PMID: 24898100
- PMCID: PMC5894862
- DOI: 10.1007/s10620-014-3230-3
Use of tenofovir disoproxil fumarate in highly viremic, hepatitis B mono-infected pregnant women
Abstract
Background: Antiviral therapy in addition to immunoprophylaxis at birth has been shown to further reduce perinatal transmission of hepatitis B virus (HBV) in highly viremic women.
Aims: The aim of this study was to describe the use of tenofovir disoproxil fumarate (TDF) prophylaxis to reduce maternal HBV DNA levels and potentially vertical transmission in highly viremic women.
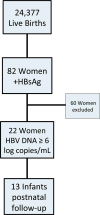
Methods: After receiving IRB approval, we performed a retrospective chart review of mothers positive for hepatitis B surface antigen (HBsAg) who delivered between 2009 and 2012. We identified women with HBV DNA levels ≥6 log copies/mL who were treated with TDF in pregnancy.
Results: There were 22 women identified. The majority were of Micronesian ethnicity. All were negative for hepatitis C antibody and HIV infection. The median gestational age of TDF initiation was 31 weeks with a median duration of treatment of 45 days. There was a reduction in median HBV DNA levels from baseline 9.0 ± 2.0 to 5.4 ± 1.1 log copies/mL after treatment. There were five (22.7 %) preterm deliveries and five (22.7 %) cesarean deliveries. All infants received immunoprophylaxis at birth. Postnatal HBsAg testing at 9-12 months was available for 13 infants, 12 of which were negative. There was one case of perinatal transmission.
Conclusions: This is the second published case series to date on the use of TDF prophylaxis in HBV mono-infected, highly viremic mothers. This series suggests the use of TDF in pregnancy reduces maternal HBV DNA levels and is well tolerated.
Conflict of interest statement
References
-
- World Health Organization. [Accessed October 9, 2013];Hepatitis B fact sheet. 2013 http://www.who.int/mediacentre/factsheets/fs204/en/
-
- Center for Disease Control and Prevention. [Accessed October 9, 2011];Viral hepatitis statistics and surveillance. 2011 http://www.cdc.gov/hepatitis/Statistics/index.htm.
-
- Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis B surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics. 2003;111:1192–1197. - PubMed
-
- Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682–1683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical