Activating receptor NKG2D targets RAE-1-expressing allogeneic neural precursor cells in a viral model of multiple sclerosis
- PMID: 24898518
- PMCID: PMC4165828
- DOI: 10.1002/stem.1760
Activating receptor NKG2D targets RAE-1-expressing allogeneic neural precursor cells in a viral model of multiple sclerosis
Abstract
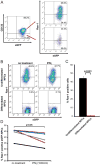
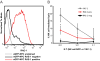
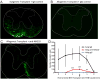
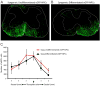
Transplantation of major histocompatibility complex-mismatched mouse neural precursor cells (NPCs) into mice persistently infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) results in rapid rejection that is mediated, in part, by T cells. However, the contribution of the innate immune response to allograft rejection in a model of viral-induced neurological disease has not been well defined. Herein, we demonstrate that the natural killer (NK) cell-expressing-activating receptor NKG2D participates in transplanted allogeneic NPC rejection in mice persistently infected with JHMV. Cultured NPCs derived from C57BL/6 (H-2(b) ) mice express the NKG2D ligand retinoic acid early precursor transcript (RAE)-1 but expression was dramatically reduced upon differentiation into either glia or neurons. RAE-1(+) NPCs were susceptible to NK cell-mediated killing whereas RAE-1(-) cells were resistant to lysis. Transplantation of C57BL/6-derived NPCs into JHMV-infected BALB/c (H-2(d) ) mice resulted in infiltration of NKG2D(+) CD49b(+) NK cells and treatment with blocking antibody specific for NKG2D increased survival of allogeneic NPCs. Furthermore, transplantation of differentiated RAE-1(-) allogeneic NPCs into JHMV-infected BALB/c mice resulted in enhanced survival, highlighting a role for the NKG2D/RAE-1 signaling axis in allograft rejection. We also demonstrate that transplantation of allogeneic NPCs into JHMV-infected mice resulted in infection of the transplanted cells suggesting that these cells may be targets for infection. Viral infection of cultured cells increased RAE-1 expression, resulting in enhanced NK cell-mediated killing through NKG2D recognition. Collectively, these results show that in a viral-induced demyelination model, NK cells contribute to rejection of allogeneic NPCs through an NKG2D signaling pathway.
Keywords: Autoimmune disease; NK cells; Nervous system; Neural differentiation; Neural stem cells; Stem cell transplantation; antigenicity.
© 2014 AlphaMed Press.
Conflict of interest statement
Potential Conflict of Interest: L.L.L. and University of California (San Francisco, CA) has licensed intellectual property rights relating to NKG2D for potential therapeutic development.
Figures



References
-
- Markovic-Plese S, Pinilla C, Martin R. The initiation of the autoimmune response in multiple sclerosis. Clin Neurol Neurosurg. 2004;106:218–222. - PubMed
-
- De Stefano N, Matthews PM, Fu L, et al. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121(Pt 8):1469–1477. - PubMed
-
- Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

