Structural and functional characterization of MERS coronavirus papain-like protease
- PMID: 24898546
- PMCID: PMC4051379
- DOI: 10.1186/1423-0127-21-54
Structural and functional characterization of MERS coronavirus papain-like protease
Abstract
Backgrounds: A new highly pathogenic human coronavirus (CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), has emerged in Jeddah and Saudi Arabia and quickly spread to some European countries since September 2012. Until 15 May 2014, it has infected at least 572 people with a fatality rate of about 30% globally. Studies to understand the virus and to develop antiviral drugs or therapy are necessary and urgent. In the present study, MERS-CoV papain-like protease (PLpro) is expressed, and its structural and functional consequences are elucidated.
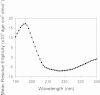
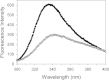
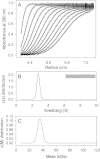
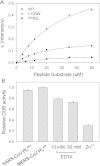
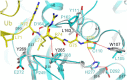
Results: Circular dichroism and Tyr/Trp fluorescence analyses indicated that the secondary and tertiary structure of MERS-CoV PLpro is well organized and folded. Analytical ultracentrifugation analyses demonstrated that MERS-CoV PLpro is a monomer in solution. The steady-state kinetic and deubiquitination activity assays indicated that MERS-CoV PLpro exhibits potent deubiquitination activity but lower proteolytic activity, compared with SARS-CoV PLpro. A natural mutation, Leu105, is the major reason for this difference.
Conclusions: Overall, MERS-CoV PLpro bound by an endogenous metal ion shows a folded structure and potent proteolytic and deubiquitination activity. These findings provide important insights into the structural and functional properties of coronaviral PLpro family, which is applicable to develop strategies inhibiting PLpro against highly pathogenic coronaviruses.
Figures

References
-
- Eckerle I, Muller MA, Kallies S, Gotthardt DN, Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. - DOI - PMC - PubMed
-
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H, Alhakeem R, Al-Tawfiq JA, Cotten M, Watson SJ, Kellam P, Zumla AI, Memish ZA, Team KM-CI. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

