Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial
- PMID: 24898552
- PMCID: PMC5994826
- DOI: 10.1093/eurheartj/ehu209
Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial
Abstract
Aim: The aim of this study was to determine long-term results of renal artery denervation for treatment of treatment-resistant hypertension in the SYMPLICITY HTN-2 study.
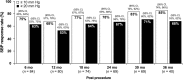
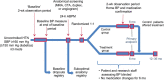
Methods: SYMPLICITY HTN-2 randomized 106 subjects with treatment-resistant hypertension to renal denervation or medical therapy alone. At 6 months, 37 control subjects crossed over to renal denervation. Office blood pressure measurements, antihypertensive medication use, and safety events were followed every 6 months through 3 years.
Results: Follow-up was available at 36 months in 40 of 52 subjects in the initial renal denervation group and at 30 months in 30 of 37 subjects who crossed over and received renal denervation at 6 months. Baseline blood pressure was 184 ± 19/99 ± 16 mmHg in all treated subjects. At 30-month post-procedure, systolic blood pressure decreased 34 mmHg (95% CI: -40, -27, P < 0.01) and diastolic blood pressure decreased 13 mmHg (95% CI: -16, -10, P < 0.01). The systolic and diastolic blood pressure reduction at 36 months for the initial renal denervation group was -33 mmHg (95% CI: -40, -25, P < 0.01) and -14 mmHg (95% CI: -17, -10, P < 0.01), respectively. Procedural complications included one haematoma, and one renal artery dissection before energy delivery that was treated successfully. Later complications included two cases of acute renal failure, which fully resolved, 15 hypertensive events requiring hospitalization, and three deaths.
Conclusion: Renal denervation resulted in sustained lowering of blood pressure at 3 years in a selected population of subjects with severe, treatment-resistant hypertension without serious safety concerns.
Clinical trial registration: NCT00888433.
Keywords: Renal denervation; Resistant hypertension; Symplicity.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2014. For permissions please email: journals.permissions@oup.com.
Figures

References
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. - PubMed
-
- Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. - PubMed
-
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. - PubMed
-
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. - PubMed
-
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM American Heart Association Professional Education C. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

