Cystic fibrosis transmembrane conductance regulator dysfunction in VIP knockout mice
- PMID: 24898584
- PMCID: PMC4101624
- DOI: 10.1152/ajpcell.00293.2013
Cystic fibrosis transmembrane conductance regulator dysfunction in VIP knockout mice
Abstract
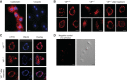
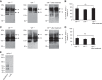
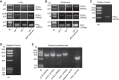
Vasoactive intestinal peptide (VIP), a neuropeptide, controls multiple functions in exocrine tissues, including inflammation, and relaxation of airway and vascular smooth muscles, and regulates CFTR-dependent secretion, which contributes to mucus hydration and local innate defense of the lung. We had previously reported that VIP stimulates the VPAC1 receptor, PKCϵ signaling cascade, and increases CFTR stability and function at the apical membrane of airway epithelial cells by reducing its internalization rate. Moreover, prolonged VIP stimulation corrects the molecular defects associated with F508del, the most common CFTR mutation responsible for the genetic disease cystic fibrosis. In the present study, we have examined the impact of the absence of VIP on CFTR maturation, cellular localization, and function in vivo using VIP knockout mice. We have conducted pathological assessments and detected signs of lung and intestinal disease. Immunodetection methods have shown that the absence of VIP results in CFTR intracellular retention despite normal expression and maturation levels. A subsequent loss of CFTR-dependent chloride current was measured in functional assays with Ussing chamber analysis of the small intestine ex vivo, creating a cystic fibrosis-like condition. Interestingly, intraperitoneal administration of VIP corrected tissue abnormalities, close to the wild-type phenotype, as well as associated defects in the vital CFTR protein. The results show in vivo a primary role for VIP chronic exposure in CFTR membrane stability and function and confirm in vitro data.
Keywords: CFTR; VIP; VIP-knockout mice; cystic fibrosis; epithelium.
Copyright © 2014 the American Physiological Society.
Figures





References
-
- Alcolado N, Conrad DJ, Rafferty S, Chappe FG, Chappe VM. VIP-dependent increase in F508del-CFTR membrane localization is mediated by PKCε. Am J Physiol Cell Physiol 301: C53–C65, 2011 - PubMed
-
- Alessandrini F, Thakkar M, Foda HD, Said SI, Lodi R, Pakbaz H, Schraufnagel DE. Vasoactive intestinal peptide enhances lung preservation. Transplantation 56: 964–973, 1993 - PubMed
-
- Ameen NA, Ardito T, Kashgarian M, Marino CR. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 108: 1016–1023, 1995 - PubMed
-
- Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J, McLaughlin GE. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 - PubMed
-
- Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

