Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2α and protein kinase A
- PMID: 24898587
- PMCID: PMC4121586
- DOI: 10.1152/ajpcell.00135.2014
Chronic opioids regulate KATP channel subunit Kir6.2 and carbonic anhydrase I and II expression in rat adrenal chromaffin cells via HIF-2α and protein kinase A
Abstract
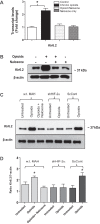
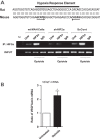
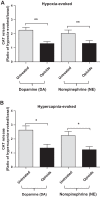
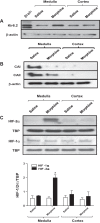
At birth, asphyxial stressors such as hypoxia and hypercapnia are important physiological stimuli for adrenal catecholamine release that is critical for the proper transition to extrauterine life. We recently showed that chronic opioids blunt chemosensitivity of neonatal rat adrenomedullary chromaffin cells (AMCs) to hypoxia and hypercapnia. This blunting was attributable to increased ATP-sensitive K(+) (KATP) channel and decreased carbonic anhydrase (CA) I and II expression, respectively, and involved μ- and δ-opioid receptor signaling pathways. To address underlying molecular mechanisms, we first exposed an O2- and CO2-sensitive, immortalized rat chromaffin cell line (MAH cells) to combined μ {[d-Arg(2),Ly(4)]dermorphin-(1-4)-amide}- and δ ([d-Pen(2),5,P-Cl-Phe(4)]enkephalin)-opioid agonists (2 μM) for ∼7 days. Western blot and quantitative real-time PCR analysis revealed that chronic opioids increased KATP channel subunit Kir6.2 and decreased CAII expression; both effects were blocked by naloxone and were absent in hypoxia-inducible factor (HIF)-2α-deficient MAH cells. Chronic opioids also stimulated HIF-2α accumulation along a time course similar to Kir6.2. Chromatin immunoprecipitation assays on opioid-treated cells revealed the binding of HIF-2α to a hypoxia response element in the promoter region of the Kir6.2 gene. The opioid-induced regulation of Kir6.2 and CAII was dependent on protein kinase A, but not protein kinase C or calmodulin kinase, activity. Interestingly, a similar pattern of HIF-2α, Kir6.2, and CAII regulation (including downregulation of CAI) was replicated in chromaffin tissue obtained from rat pups born to dams exposed to morphine throughout gestation. Collectively, these data reveal novel mechanisms by which chronic opioids blunt asphyxial chemosensitivity in AMCs, thereby contributing to abnormal arousal responses in the offspring of opiate-addicted mothers.
Keywords: adenosine 5′-triphosphate-sensitive potassium channels; carbonic anhydrase; hypoxia-inducible factor-2α; opioid.
Copyright © 2014 the American Physiological Society.
Figures





Similar articles
-
Chronic exposure of neonatal rat adrenomedullary chromaffin cells to opioids in vitro blunts both hypoxia and hypercapnia chemosensitivity.J Physiol. 2013 Jan 15;591(2):515-29. doi: 10.1113/jphysiol.2012.243477. Epub 2012 Nov 12. J Physiol. 2013. PMID: 23148319 Free PMC article.
-
Chronic nicotine induces hypoxia inducible factor-2α in perinatal rat adrenal chromaffin cells: role in transcriptional upregulation of KATP channel subunit Kir6.2.Am J Physiol Cell Physiol. 2012 May 15;302(10):C1531-8. doi: 10.1152/ajpcell.00052.2012. Epub 2012 Mar 7. Am J Physiol Cell Physiol. 2012. PMID: 22403787
-
Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha.J Neurosci. 2009 Jun 3;29(22):7137-47. doi: 10.1523/JNEUROSCI.0544-09.2009. J Neurosci. 2009. PMID: 19494136 Free PMC article.
-
Ontogeny of O2 and CO2//H+ chemosensitivity in adrenal chromaffin cells: role of innervation.J Exp Biol. 2014 Mar 1;217(Pt 5):673-81. doi: 10.1242/jeb.086165. J Exp Biol. 2014. PMID: 24574383 Review.
-
Opioidergic and dopaminergic modulation of respiration.Respir Physiol Neurobiol. 2008 Dec 10;164(1-2):160-7. doi: 10.1016/j.resp.2008.02.004. Respir Physiol Neurobiol. 2008. PMID: 18394974 Free PMC article. Review.
Cited by
-
Hypoxia-regulated catecholamine secretion in chromaffin cells.Cell Tissue Res. 2018 May;372(2):433-441. doi: 10.1007/s00441-017-2703-z. Epub 2017 Oct 19. Cell Tissue Res. 2018. PMID: 29052004 Review.
-
Gene expression analyses reveal metabolic specifications in acute O2 -sensing chemoreceptor cells.J Physiol. 2017 Sep 15;595(18):6091-6120. doi: 10.1113/JP274684. Epub 2017 Aug 8. J Physiol. 2017. PMID: 28718507 Free PMC article.
-
Localization of the delta opioid receptor and corticotropin-releasing factor in the amygdalar complex: role in anxiety.Brain Struct Funct. 2017 Mar;222(2):1007-1026. doi: 10.1007/s00429-016-1261-6. Epub 2016 Jul 4. Brain Struct Funct. 2017. PMID: 27376372 Free PMC article.
-
Hypoxia-dependent signaling in perioperative and critical care medicine.J Anesth. 2021 Oct;35(5):741-756. doi: 10.1007/s00540-021-02940-w. Epub 2021 May 18. J Anesth. 2021. PMID: 34003375 Free PMC article. Review.
References
-
- Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol 28: 3386–3400, 2008 - PMC - PubMed
-
- Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem 110: 622–630, 2009 - PubMed
-
- Brown ST, Nurse CA. Induction of HIF-2alpha is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line. Am J Physiol Cell Physiol 294: C1305–C1312, 2008 - PubMed
-
- Burns L, Conroy E, Mattick RP. Infant mortality among women on a methadone program during pregnancy. Drug Alcohol Rev 29: 551–556, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

