Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein
- PMID: 24898843
- PMCID: PMC4121631
- DOI: 10.1152/ajpregu.00116.2014
Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein
Abstract
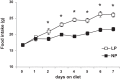
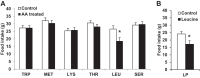
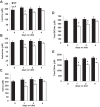
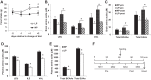
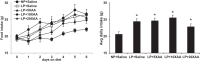
Intracerebroventricular injections of leucine are sufficient to suppress food intake, but it remains unclear whether brain leucine signaling represents a physiological signal of protein balance. We tested whether variations in dietary and circulating levels of leucine, or all three branched-chain amino acids (BCAAs), contribute to the detection of reduced dietary protein. Of the essential amino acids (EAAs) tested, only intracerebroventricular injection of leucine (10 μg) was sufficient to suppress food intake. Isocaloric low- (9% protein energy; LP) or normal- (18% protein energy) protein diets induced a divergence in food intake, with an increased consumption of LP beginning on day 2 and persisting throughout the study (P < 0.05). Circulating BCAA levels were reduced the day after LP diet exposure, but levels subsequently increased and normalized by day 4, despite persistent hyperphagia. Brain BCAA levels as measured by microdialysis on day 2 of diet exposure were reduced in LP rats, but this effect was most prominent postprandially. Despite these diet-induced changes in BCAA levels, reducing dietary leucine or total BCAAs independently from total protein was neither necessary nor sufficient to induce hyperphagia, while chronic infusion of EAAs into the brain of LP rats failed to consistently block LP-induced hyperphagia. Collectively, these data suggest that circulating BCAAs are transiently reduced by dietary protein restriction, but variations in dietary or brain BCAAs alone do not explain the hyperphagia induced by a low-protein diet.
Keywords: branched-chain amino acids; food intake; hypothalamus; macronutrient; protein restriction.
Copyright © 2014 the American Physiological Society.
Figures
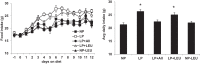
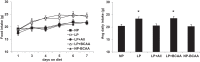
References
-
- Anderson SA, Tews JK, Harper AE. Dietary branched-chain amino acids and protein selection by rats. J Nutr 120: 52–63, 1990 - PubMed
-
- Bensaid A, Tome D, L'Heureux-Bourdon D, Even P, Gietzen D, Morens C, Gaudichon C, Larue-Achagiotis C, Fromentin G. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav 78: 311–320, 2003 - PubMed
-
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

