HPTN 035 phase II/IIb randomised safety and effectiveness study of the vaginal microbicides BufferGel and 0.5% PRO 2000 for the prevention of sexually transmitted infections in women
- PMID: 24898857
- PMCID: PMC4278566
- DOI: 10.1136/sextrans-2014-051537
HPTN 035 phase II/IIb randomised safety and effectiveness study of the vaginal microbicides BufferGel and 0.5% PRO 2000 for the prevention of sexually transmitted infections in women
Abstract
Objectives: To estimate the effectiveness of candidate microbicides BufferGel and 0.5% PRO 2000 Gel (P) (PRO 2000) for prevention of non-ulcerative sexually transmitted infections (STIs).
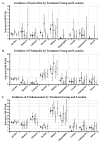
Methods: Between 2005 and 2007, 3099 women were enrolled in HIV Prevention Trials Network (HPTN) protocol 035, a phase II/IIb evaluation of the safety and effectiveness of BufferGel and PRO 2000 for prevention of STIs, including Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT) and Trichomonas vaginalis (TV). Incidences of STIs were determined by study arm, and HRs of BufferGel and PRO 2000 versus placebo gel or no gel control groups were computed using discrete time Andersen-Gill proportional hazards model.
Results: The overall incidence rates were 1.6/100 person-years at risk (PYAR) for NG, 3.9/100 PYAR for CT and 15.3/100 PYAR for TV. For BufferGel versus placebo gel, HRs were 0.99 (95% CI 0.49 to 2.00), 1.00 (95% CI 0.64 to 1.57) and 0.95 (95% CI 0.71 to 1.25) for prevention of NG, CT and TV, respectively. For PRO 2000, HRs were 1.66 (95% CI 0.90 to 3.06), 1.16 (95% CI 0.76 to 1.79) and 1.18 (95% CI 0.90 to 1.53) for prevention of NG, CT and TV, respectively.
Conclusions: The incidence of STIs was high during HIV Prevention Trials Network 035 despite provision of free condoms and comprehensive risk-reduction counselling, highlighting the need for effective STI prevention programmes in this population. Unfortunately, candidate microbicides BufferGel and PRO2000 had no protective effect against gonorrhoea, chlamydia or trichomoniasis.
Trial registration number: NCT00074425.
Keywords: CHLAMYDIA TRACHOMATIS; MICROBICIDES; NEISSERIA GONORRHOEA; PREVENTION; TRICHOMONAS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
None of the authors, or their immediate family, has any specified relationships with any companies that might have an interest in the submitted work. Among the remaining members of the HPTN 035 Study Team, A.P. was an employee of, and held equity interest in, Endo Pharmaceuticals Solutions, the owner of PRO 2000. T.M. was an employee of ReProtect that sponsored BufferGel for the study, and R.B., L.S.-T., and S.E. were employed by the NIH, which funded the study.
Figures
References
-
- World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. Geneva: 2007.
-
- Pinkerton SD, Layde PM. Using sexually transmitted disease incidence as a surrogate marker for HIV incidence in prevention trials: a modeling study. Sex Transm Dis. 2002;29(5):298–307. - PubMed
-
- Plummer FA, Simonsen JN, Cameron DW, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163(2):233–9. - PubMed
-
- Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7(1):95–102. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
