Impact of renal medullary three-dimensional architecture on oxygen transport
- PMID: 24899054
- PMCID: PMC4121569
- DOI: 10.1152/ajprenal.00149.2014
Impact of renal medullary three-dimensional architecture on oxygen transport
Abstract
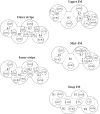
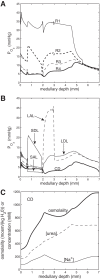
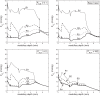
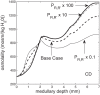
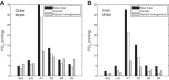
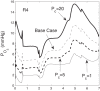
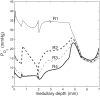
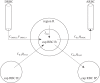
We have developed a highly detailed mathematical model of solute transport in the renal medulla of the rat kidney to study the impact of the structured organization of nephrons and vessels revealed in anatomic studies. The model represents the arrangement of tubules around a vascular bundle in the outer medulla and around a collecting duct cluster in the upper inner medulla. Model simulations yield marked gradients in intrabundle and interbundle interstitial fluid oxygen tension (PO2), NaCl concentration, and osmolality in the outer medulla, owing to the vigorous active reabsorption of NaCl by the thick ascending limbs. In the inner medulla, where the thin ascending limbs do not mediate significant active NaCl transport, interstitial fluid composition becomes much more homogeneous with respect to NaCl, urea, and osmolality. Nonetheless, a substantial PO2 gradient remains, owing to the relatively high oxygen demand of the inner medullary collecting ducts. Perhaps more importantly, the model predicts that in the absence of the three-dimensional medullary architecture, oxygen delivery to the inner medulla would drastically decrease, with the terminal inner medulla nearly completely deprived of oxygen. Thus model results suggest that the functional role of the three-dimensional medullary architecture may be to preserve oxygen delivery to the papilla. Additionally, a simulation that represents low medullary blood flow suggests that the separation of thick limbs from the vascular bundles substantially increases the risk of the segments to hypoxic injury. When nephrons and vessels are more homogeneously distributed, luminal PO2 in the thick ascending limb of superficial nephrons increases by 66% in the inner stripe. Furthermore, simulations predict that owing to the Bohr effect, the presumed greater acidity of blood in the interbundle regions, where thick ascending limbs are located, relative to that in the vascular bundles, facilitates the delivery of O2 to support the high metabolic requirements of the thick limbs and raises NaCl reabsorption.
Keywords: hypoxia; mathematical model; metabolism; thick ascending limb transport.
Copyright © 2014 the American Physiological Society.
Figures
References
-
- Andersson LG, Bratteby LE, Ekroth R, Hallhagen S, Joachimsson PO, Linden van der J, Wesslén O. Renal function during cardiopulmonary bypass: influence of pump flow and systemic blood pressure. Eur J Cardiothorac Surg 8: 597–602, 1994 - PubMed
-
- Aperia AC. The influence of arterial PO2 on renal tissue PO2. Acta Physiol Scand 75: 353–359, 1969 - PubMed
-
- Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985 - PubMed
-
- Bankir L, de Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985 - PubMed
-
- Baumgartl H, Leichtweiss HP, Lubbers DW, Weiss C, Huland H. The oxygen supply of the dog kidney: measurements of intrarenal pO2. Microvasc Res 4: 247–257, 1972 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

